AMAZON multi-meters discounts AMAZON oscilloscope discounts
Crystallization is a very low energy separation method for many organic mixtures.
Organic chemicals are most frequently separated by cooling crystallization which often means relatively low temperatures are involved, minimizing corrosion problems. Typical fluids include fatty acids and chlorobenzenes. High purity is normally possible in one or two steps.
CONTINUOUS CRYSTALLIZERS FOR ORGANIC CHEMICALS
Solubility Thermodynamics
The Ideal Case
There are a number of frequently encountered cases where the ideal liquid mixture assumptions are applicable, and in such cases the solubility, and therefore the ease of separation can be easily calculated. Many such systems are reaction mixtures which do not lend themselves to conventional separation methods. Some frequently encountered examples are:
- mixed xylenes
- mixed chlorobenzenes
- paraffins
- many multi-substituted benzenes
Non-ideal Case
There are a number of cases where these assumptions are not true, these include:
++Polar solutes in polar solvents such as fatty acids in acetone
++Polarizable solutes in polar solvents, such as naphthalene in methanol
++Dimerization or hydrogen bonding, such as many organic acids
Prediction of Solubility
Under those conditions where the assumptions of ideality can be considered to hold, the solubility relationship is quite simple. In the ideal case, the solubility curves and eutectics can be fairly accurately predicted by the Van T'Hoff equation:
- Xa is the mole fraction in solution of component a
- Xa is the molar heat of fusion of component a
- R is the gas component
- To is the melting temperature of component a at the system pressure
- T is the system temperature
Thus given the melting point of a substance and its molar heat of fusion, it is possible to predict its solubility in an ideal mixture and by judicious use of these results, predict the eutectic temperature and composition.
FIG. 1 [coming soon] Theoretical Solubility of ortho and para-dichlorobenzene. (Armstrong Engineering Associates, Inc West Chester, PA)
FIG. 1 [coming soon] shows a direct plot of the Van T'Hoff equation, relating the mole fraction of both ortho and paradichlorobenzene in solution in an ideal mixture at the temperatures shown. This means that for the case of an ideal mixture of para in a solvent, the composition of the saturated liquid phase is as indicated by the para curve. For instance this ideal liquid mixture would contain about 26% para at 0 degrees centigrade, regard less of solvent, so long as the system is ideal. The ortho curve has the same significance.
A mixture of ortho and para isomers is a frequently encountered case, and closely approximates the ideal predictions. In this case, mole and weight percents are identical as the two components are isomers. In this binary case, the solubility of para is read from its curve, and the ortho is the balance needed to sum to 100 percent. The balance, which is the ortho percent, must be less than or equal to the percents indicated by the ortho curve, otherwise an impossible case could arise of more than 100 percent.
At the point where the sum of the ortho and para curves equal 100 percent, the eutectic occurs, and further change of composition is impossible for that system.
There is a unique temperature where this will occur. At this point the system shows equilibrium between the liquid phase mixture of the two isomers, and solid pure para and also pure ortho as solid. The phase rule indicates this is a determinant point, and if the temperature is further lowered, solidification will take place, producing the homogeneous eutectic. As nearly as the plot can be read, this turns out to be at -24 degree with a composition of 14% para, and 86% ortho. The International Critical Tables show the eutectic to be at -23.4 degree with a composition of 86.7% ortho, and 13.3% para, which is certainly excellent agreement.
FIG. 2 [coming soon] Theoretical Solubility of para in ortho dichlorobenzene 3. (Armstrong Engineering Associates, Inc West Chester, PA)
Plotting the figure in another way, solubility expressed as grams para per 100 grams ortho will give the results most often used to calculate the recoveries, as in FIG. 2 [coming soon] . Here it is abundantly clear that the way to separate a mixture of these two components is to cool the mixture, driving the para out of solution. As the cooling approaches the eutectic point nearly total recovery is attained. A simple mass balance will show that a mixture of 65% para, 35% ortho can be reduced to the eutectic point with a theoretical recovery of around 90% of the para. At that point the mixture, ortho rich mother liquor can be sold, or perhaps the eutectic split using solvents to produce pure substances.
While the calculations shown are theoretical in nature, the results are almost indistinguishable from published solubility data. Such calculations can be quite useful for basic feasibility work to evaluate whether crystallization is a desirable alternative to more conventional separation methods. If this is the case, verifications of the predictions of the theory are usually advisable, and easy to carry out in simple laboratory equipment.
Low Energy Separation of Organic Chemicals
Many organic mixtures may be separated by cooling crystallization, as indicated in the solubility thermodynamics part of this section.
In simplest terms a cooling crystallization means that a mixture of organic chemicals is reduced in temperature, without removal (by evaporation) of any of the components. In this simple cooling operation, the heat removal consists of sensible heat, which is generally low for organics, and also the latent heat of fusion, which is also quite low.
Crystallization is a "one way" process; the heat is removed, crystals are formed, and the mixture of crystals and solids formed are then separated. This must be compared to distillation which is inherently a refluxing operation, where products are repeatedly evaporated and re-condensed.
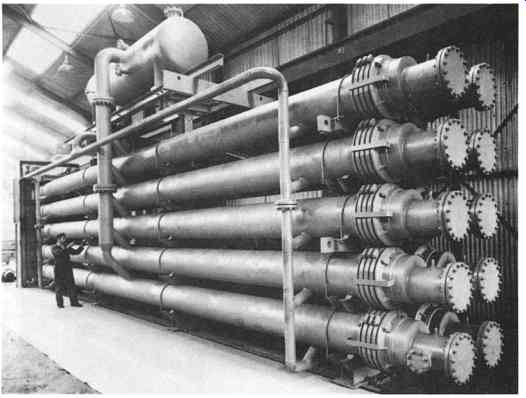
FIG. 3 Large carbon steel unit for crystallizing petroleum wax. Refrigeration
is by boiling refrigerant. (Armstrong Engineering Associates, Inc West Chester,
PA)
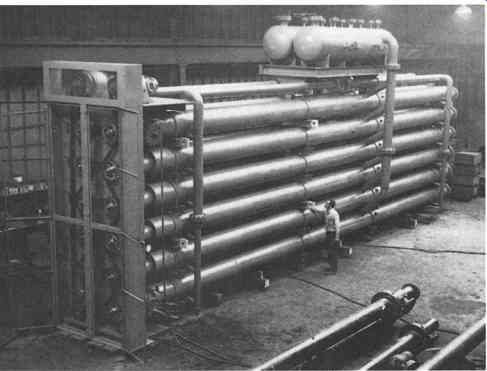
FIG. 4 Scraped surface chiller with two cooling circuits of boiling refrigerants.
One of several in the installation, surface 1680ft^2 (156 m^2) (Armstrong
Engineering Associates, Inc West Chester, PA)
The latent heat of fusion is generally much lower than the latent heat of vaporization. Since the latent heat of fusion must be removed only once, instead of many times as in distillation, the energy requirements are drastically lower for crystallization.
Most distillations take place at elevated temperatures, which means the materials being processed must be heated up, and then cooled back down again normally with energy losses each way. Also many distillations are run under vacuum to effect a better separation, however, most vacuum systems are also energy consumers on a large scale.
Many crystallizations take place at around ambient temperatures so there is little heat up or cool down required to get to the right conditions for the separation to start.
In the great majority of cases, the crystals which form are 100% pure material, as opposed to something only slightly richer than the feed material, thus it is not necessary to repeatedly melt and refreeze to obtain quite high purities. Certainly the pure crystals will have some impure mother liquor on the surfaces and sometimes contained within the crystals as occlusions. However, the purity increase is extremely rapid and normally one or perhaps two crystallizations can give very high purities.
Many of the materials which separate most easily on crystallization are in any event very difficult to separate by distillation. Examples include ortho- and para-di-substituted benzenes, fatty acids, and many others of commercial significance. In these cases extremely large distillation columns are required, with exceedingly high reflux rates, which are very energy intensive.
In many cases it is possible to arrange for energy recovery in crystallization systems by using the cold separated product to carry out part of the cooling required to obtain the crystals, thus reducing the energy required even more.
With rising energy costs, it makes sense to take a critical look at many separations to see if crystallization may be the best altemative.
Advantages of Crystallization
Utility requirements are low, as discussed in the previous section.
Low temperature operation, which means low corrosion rates, and often use of less costly alloys compared to evaporation based separations. The low temperatures utilized also mean little or no product degradation which for heat sensitive materials may be extremely important. There is no formation of "tars" which represent a yield loss, and increasingly a severe waste disposal problem, and normally require additional separation equipment (and energy) for their removal, to give desired product color, etc.
Enclosed systems, with little chance of leakage of dangerous or noxious fluids.
The systems are normally simple and require few pieces of equipment and relatively little instrumentation.
Favorable equilibrium; often freezing points are widely separated, allowing for relatively easy separation by crystallization, where distillations may be exceedingly difficult.
High purity; the crystals which form in the great majority of cases are 100% pure material. While impurities may adhere to crystal surfaces, or be included inside the crystal, re-crystallization can normally achieve very high purities, with relative ease. 95 to 99.5% are normal product purities, although higher figures are also often reached.
Crystallization can easily be made continuous; in general, the only reason to work with a batch crystallization is very low design capacity. If a high enough design capacity is desired (normally above 500,000 pounds of product annually), it is easy to make a fully continuous crystallization, with consequent savings in energy and manpower.
Crystallizer for Paraxylene
Continuous scraped surface crystallizers for paraxylene purification may be designed to use either boiling refrigerant, or brine, depending on temperature level, and the flow rates involved.
Brine cooling has a number of advantages in the higher purity, high temperature ranges, while boiling refrigerants offer advantages at lower temperatures.
There is no practical upper limit on capacity, as some of the largest plants in the world are currently using this equipment. Rugged construction and field proven design assure long run times even in very tough duties.
Process design consultation is normally involved with such facilities to assure troublefree operations.
Design and construction is available in most international pressure vessel codes, such as ASME, Stoomwezen, TUV, CODAP, ISPESL, Swedish and Australian Pressure Vessel Code, etc.
Crystallization is a very powerful separation method for obtaining high product purities, such as those demanded by current paraxylene customers. The crystals which form are inherently pure (i.e. 100% paraxylene). The only impurities are mother liquors sticking to the surface, or included in the crystal matrix, where washing can remove them.
Moderate temperatures are required in high-purity paraxylene crystallizations, so special materials, and high refrigeration loads are not involved.
Skimming is a cost-effective method to recover paraxylene from various recycle streams which are relatively rich in this isomer. Current practice often mixes such streams with other, much leaner streams, losing the relative advantages of the purity already achieved.
Crystallization facilities may be expanded on an incremental basis which allows for easy de-bottlenecking should this become attractive in the future.
Working Principles of a Surface Continuous Crystallizer
A scraped surface crystallizer is built as a double pipe element, generally with 6, 8, 10, or 12 inch inner pipe, and an outer pipe suited to the duty. The area between the inner and outer pipe is filled with cooling fluid. The inner pipe contains a rotating scraper element, shown schematically in FIG. 5 which mixes the process fluid flowing through the inner pipe, and removes deposits which may form on the wall as cooling occurs.
In effect, the scraped surface crystallizer is a heat exchanger but quite an unusual one. It generally performs as a cooling crystallizer, since the solubility thermodynamics portion of this folder indicates the high potential for cooling crystallization for organic chemicals.
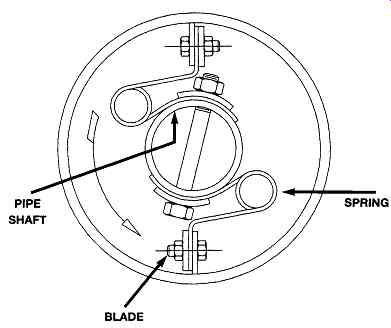
FIG. 5 Cross-section of scraped surface crystallizer. (Armstrong Engineering
Associates, Inc West Chester, PA)
Heat transfer occurs across the wall in the inner pipe, with cold fluid outside, and process fluid inside. As this cooling occurs, crystals tend to form, and foul, on the inner pipe wall. The scraper blades there rotate and remove crystalline deposits which would inhibit heat transfer. In general, the majority of the crystallization takes place in the bulk of the fluid, as opposed to the wall, thus allowing growth of easily separable crystals.
Scraper blades rotate at moderate speeds, generally 15 to 30 rpm. Higher speeds will give higher heat transfer, but the high shear which accompanies it will normally produce much smaller crystals. An extension of this is the margarine or ice cream freezer, which are designed specifically to crystallize such materials, but produce tiny, inseparable crystals. This is accomplished by use of extremely high rpm, high shear, and high heat transfer. The ice cream machine is often confused with a crystallizer, since the principle is similar, but the result very different. Crystallizers are designed specifically for adequate heat transfer, consistent with good crystal growth.
A normal commercial sized installation will consist of several double pipe elements, each with a length of 20 to 40 feet, connected in series. This gives a long thin flow path which promotes a close approach to plug flow, which is very important in many crystallizations.
Cooling is carried out by whatever coolant is selected, and run through the annulus between inner and outer pipe. Coolants may be cooling tower water, chilled brines, evaporating refrigerants, mother liquor stripped of crystals, or combinations of these.
Fabrication of the equipment is available in alloys normally found in chemical plants, such as carbon and stainless steels, nickel alloys such as inconel, Incoloy, monel, etc.
Construction is available to international pressure vessel codes such as ASME, BS5500, TI~IV, Stoomwezen, Swedish Pressure Vessel Code, Australian DLI, etc.
Many organic chemical crystallizations are done using batch cooling in stainless steel or glass-lined kettles. By and large this represents continued growth from specialty chemical to commodity, without a great deal of engineering attention to the crystallization part of the process. Continuous crystallization offers a number of advantages over batch crystallization:
(a) Smaller equipment which generally means less expensive installations and small volumes of sometimes hazardous or expensive material in process.
(b) Less floor space.
(c) Less operator labor.
(d) No duplication of instrumentation, piping, etc.
(e) Less upsets, or peak utility demand.
(f) Better process control.
Advantages of Scraped Surface Crystallizers
Many continuous crystallizations are done in evaporative or cooling crystallizers based on designs typically used for inorganic chemicals where there is normally a very fiat solubility curve, i.e., a change in mixture temperature produces relatively few crystals. Other are sometimes done by cooling and partially crystallizing in shell and tube exchangers.
Scraped surface continuous crystallizers offer advantages as follows:
May be run for extended periods between hot washing where many shell and tube exchangers would plug up in minutes.
May be run at much higher temperature differences between process fluid and coolant than may ever be attempted with shell and tube equipment, without serious fouling, or plugging.
May be used over an extremely wide temperature range, from -75 degree to 4-100 degree It is normally very difficult to run vacuum crystallization equipment over a broad range of temperatures.
Can be used with a high percentage of solids. Vacuum crystallizers are normally limited to about 25% by weight or less solids, while we have worked into the range of 65% by weight solids as slurry.
High viscosities are not a major problem, with several crystallizations being carried out from mother liquors with viscosities of 10,000 cp or higher.
Flow pattern in once-through operation closely resembles plug flow so conversion of batch to continuous processes is easy, and virtually any time/temperature pattern desired is possible.
In small throughput cases, a scraped surface crystallizer will be very inexpensive, even where in much larger installations a vacuum crystallization may be most attractive.
Modular construction allows for very easy expansion with growth in demand.
Simple, self-contained construction, with minimum instrumentation, and auxiliaries such as condensers, vacuum systems, etc.
Applications
For scraped surface cooling crystallizations
Good Solubility Curve
Cooling crystallizations are obviously most suitable where the solubility curve will give good yields with simple cooling of the mixture. This is true of a wide variety of organic mixtures.
Low Temperature Crystallizations
Scraped surface crystallizers offer the best approach to low temperature crystallization as, for example, the separation of meta and para-xylenes, or oleic and linoleic acids.
Products with High Boiling Point Rise
Some mixtures of inorganic chemicals in water show very high boiling point rises as concentration proceeds, reducing the vapor pressure, and much increasing the vacuum requirement. Many such mixes produce abundant crystal growth on cooling.
Often a scraped surface unit may be used in conjunction with a vacuum unit, with a vacuum unit doing the high temperature part of the crystallization and a scraped surface unit doing the low temperature part.
Products with Similar Vapor Pressures
Many aromatic chemicals, particularly isomers, have nearly identical vapor pressure characteristics, making distillation very difficult. These same mixes often have widely varying freezing points, making crystallization simple, and effective.
High Viscosity Fluids
High viscosity, due either to high mother liquor viscosity or a high percentage of solids do not present problems to the scraped surface crystallizer but may make other types of crystallizers totally inoperable.
Severe Fouling
The fouling tendencies of many slurries are opposed, and deposits on the heat transfer surfaces continuously removed.
---
Partial of List Products Crystallized
anthracene anthraquinone naphthalene nitrochlorobenzene benzene hexachloride benzoic acid bisphenol A butyl cresol butyric acid caffeine calcium nitrate caprolactam cyanoacetamide dibutyl cresol diglycerides dimethyl hydantoin dimethyl terephthalate fatty acids oligomers palm/palm kernel fats paracresol paradichlorobenzene paraxylene pentaerythritol potassium chloride potassium nitrate sebacic acid silver nitrate sodium carbonate sodium lauryl sulfate sodium sulfate sorbic acid sterols lactose laurolactam levulinic acid menthol methionine monoglycerides tall oil fatty acids tallow fatty acids tetrachlorobenzene tetramethyl benzene vitamins waxes
---
Separation of Chiorobenzenes
Para and ortho di-chlorobenzene, which were used in the example on solubility thermodynamics, represent two important chemical products which lend themselves to separation by cooling crystallization. The para-isomer crystallizes at temperatures far above the point where either ortho crystals, or the eutectic is reached. Para dichlorobenzene forms extremely tough crystals, which adhere readily to any cooled surface, requiring vigorous scraping to remove them. The tough crystals can stand a certain amount of abuse without degradation in size.
The mixture normally worked with produces a very thick slurry, and great care must be exercised handling it. The extremely steep solubility curve presents many opportunities for crystal growth, as well as dangers of uncontrolled crystallization, and must be handled carefully or the whole unit may freeze solid.
Strong equipment, and ingenious slurry handling, often with staged operations, are the basics of this process, and similar separations of xylene isomers, cresols, and other di-substituted benzenes.
Separation of Fatty Materials
Fatty acids from tallow or tall oil, mono-, di-, triglycerides, fatty alcohols, and related compounds all may be separated by crystallization where other separation means will not work. These separations all must take into account the extremely delicate nature of the crystal, and the sensitivity to shear which can rapidly produce an inseparable crystal.
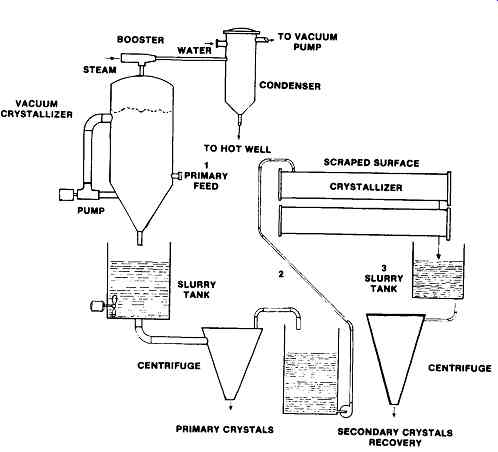
FIG. 6 Secondary recovery by a scraped surface crystallizer. (Armstrong
Engineering Associates, Inc West Chester, PA)
The time/temperature relationship is of extreme importance as well, sometimes requiring sophisticated cooling arrangements on the shell sides of the equipment.
Solvents are sometimes used to obtain optimal separations, although solvent free separations using detergents to separate saturated and unsaturated compounds have also frequently been used.
Typically, crystal growth is relatively slow and care must be exercised to allow time to grow a decent crystal, which may be easily separated. Reducing shear is more important than producing a rugged machine for these delicate materials.
Secondary Recovery
An opportunity exists to recover additional product from waste streams from vacuum or other crystallizers due to the better cooling capability of scraped surface crystallizers.
In many cases, either due to boiling point rise or temperature characteristics, vacuum crystallizers can only cool so far, for example to say 35 to 40 degree as the steam consumption begins to get extremely high for the amount of vapor removed.
With high steam consumption goes high cooling water requirements as well, plus large capital equipment needs.
Scraped surface crystallizers can be refrigerated easily and can cool as low as minus 100 degree without great difficulty and therefore can easily cool the effluent from the vacuum crystallizer to effect additional recovery of product beyond that which can economically be obtained in the vacuum crystallizer. It is possible the recovered product can quickly pay for the additional equipment, also it is an easy way to increase output without otherwise increasing capital equipment.
The solubility chart (FIG. 7) illustrates a typical solution. The normal feed to the vacuum crystallizer enters at (1) and is cooled to (2). The temperature at that point is the economic limit, either due to being a low temperature, or being the result of high boiling point rise. Therefore it is either impossible or highly uneconomical to carry the process further with vacuum equipment. There are many such situations in existence today.
The scraped surface crystallizer can cool from point (2) down to point (3), effecting appreciable further recovery of crystals because it can easily reduce the temperature, and consequently lower the solubility.
Other Applications of Scraped Surface Exchangers
While the vast majority of scraped surface exchangers are in crystallization, the equipment is sufficiently versatile that a wide range of process problems have been successfully solved by use of scraped surface exchangers.
Crystallization of Inorganics
Many inorganic chemicals are crystallized from aqueous solutions on a massive scale. Typically, however, this is done by evaporative crystallization as the solubility curve is extremely fiat, so cooling crystallization will not produce many crystals.
There are, however, some important exceptions. Sodium sulphate, sodium carbonate, potassium nitrate are all often encountered in quantities where cooling crystallization has merit, and have been successfully handled in scraped surface crystallizers.
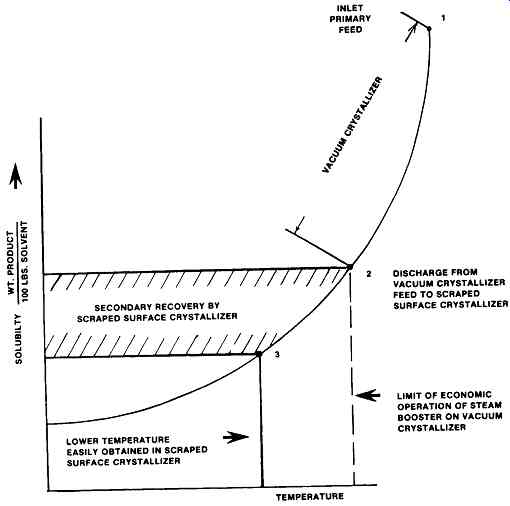
FIG. 7 Solubility diagram of Secondary Recovery. (Armstrong Engineering
Associates, Inc West Chester, PA)
Cooling Viscous Liquids (to 100,000 cp)
There are many instances where heat exchange is difficult because of the viscosity of a process stream. In such cases, Armstrong scraped surface heat exchangers have shown their merit, handling materials with up to 100,000 centipoises viscosity with high heat transfer. Added benefits of scraped surface exchangers in such duties are the inherently low pressure drops of this type of equipment, and the ability to remove any sticky foulant which might from such viscous fluids.
Scraped Reactor Coolers
Scraped surface exchangers have demonstrated their ability to handle high viscosity, fouling fluids, producing good heat transfer, and near plug flow.
These unique characteristics make it a very effective reactor for a wide variety of exothermic reactions. Ease of temperature control adds further important flexibility.
Sublimation Condensing
Condensation of a vapor as a solid, or de-subliming, is another area where scraped surface exchangers have proven their value. In such cases it is necessary to continuously remove a fouling film and allow the solid condensate to be carried away from the unit, while permitting the process stream to pass through. There are a wide number of organic chemical reactions where an off gas contains a valuable component which can be recovered by the use of a scraped surface condenser, but where a normal condenser would rapidly foul with solid condensate. Such condensers also find use in purification operations where a flash distillation separates components and purifies the condensate, which condenses as a solid.
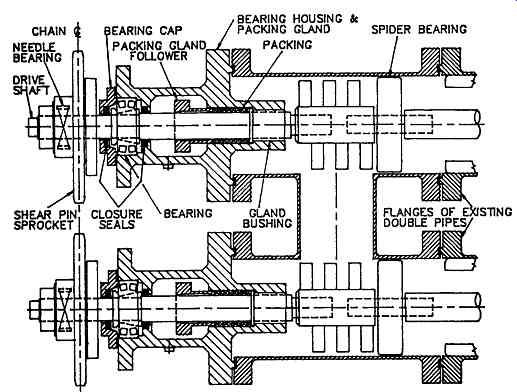
FIG. 8 Replacement drive-all bolted, no hot work required. (Armstrong Engineering
Associates, Inc West Chester, PA)
SCRAPED SURFACE DEWAXING EXCHANGERS AND CHILLERS
In many cases MEK dewaxing units are in basically good condition with the exception of the scraped surface dewaxing exchangers and chillers. In today's world, it is often difficult to justify putting up a new grass roots lube facility, however, rebuilding existing plants offers a number of advantages. Far lower capital costs are involved, timing is very short, and there may be no environmental approvals required. At the same time, substantial upgrades of equipment are possible, improving process and mechanical performance. As in many other fields, the scraped surface dewaxing equipment designed today is considerably better than that made 30 or 50 years ago. Fortunately, retrofits are easily possible for such important parts as the scraper internals, shear pin sprockets, drive ends, and in some cases, the double pipes themselves. This allows the reuse of some of the existing equipment, but upgrading of the wear parts to current practice.
The scraper internal is the heart of the scraped surface exchanger or chiller.
It must run for a number of years without maintenance, or even the possibility of examination. As moving parts, there will be wear on the scraper internals. Although installed in a lubricating oil plant, the solvents cut any lubricating qualities of the charge stock so the internals work in an environment which is likely to cause rapid wear. To counter this, only the best materials of construction, fabrication tolerances, and design approaches can be depended on to give maximum life.
As wear parts, the scraper internals must be replaced from time to time. This gives the opportunity to retrofit state of the art internals on a normal maintenance basis.
Next to the scraper internals, the drive mechanism is typically the part of the scraped surface exchanger which causes most maintenance headaches. Armstrong have for many years been involved in the business of replacing the originally furnished drive mechanism with drive trains as illustrated above. In such a replacement, a return bend is frequently removed and replaced. With a fabricated crossover which would bolt to the inner pipe. The drive end itself is in turn bolted to this.
The drive mechanism is designed to be extremely compact, but rugged. In operation it has proven to be very reliable, and easy for mechanics to understand. While an overhung drive shaft is used, the thickness of the shaft and short dimensions have produced a reliable drive which has caused virtually no problems in operation.
In changing over to this type of drive, it is normally necessary to send an engineer to the site to measure exactly what is required, as often old drawings are lacking in sufficient detail to make such a changeover. While this is expensive, it does save problems of things not fitting properly which can cause expensive last minute modifications which have led to mistakes being made in a number of cases, where local machine shops have been asked to do similar work. It is necessary to understand the function of the drive to design alterations.
Mechanical Seals
Environmental constraints have increasingly indicated that less leakage from stuffing boxes is highly desirable. Some installations have opted for the use of mechanical seals to minimize leakage at the entry point for the drive shaft. This is not inexpensive, but it has cut leakage drastically which has paid off in reduced solvent losses, solvent attack of the drive, and of course reduced air pollution. Such drives are available as replacements to existing stuffing box drive ends.
Some instances of overhaul of old equipment have involved rebuilding the double pipe elements. There have been many variations on just how this has been carried out. In some cases the "carcass" of the double pipes alone has been fabricated and shipped out, to be fit on location with drive and internals, etc. In other cases the whole exchanger has been removed pulled apart and the inner pipe, drive and internals replaced or variations made as needed.
In a number of cases it has been decided to merely scrap the existing equipment and purchase totally new exchangers and chillers, but with the provision that they fit on the existing foundations, and meet existing piping.
Most scraped surface exchangers or chillers are fit with a shear pin to protect the scraper internals in the event of something becoming lodged in them. Traditionally these have been made with a sleeve bearing between the halves of the sprocket.
When the shear pin breaks, the inner part remains stationary while the outer part rotates with the driving chain. This causes some wear on the sleeve bearing. The wear allows a slight wobble which can cause premature failure of the shear pin, in turn causing more sleeve bearing wear, etc.
This problem has been eliminated by the Armstrong shear pin sprocket. An antifriction needle bearing has been fitted between the halves of the shear pin sprocket. Upon failure of the shear pin the outer part rotates but there is no bearing wear. This is true for extended running periods - once nine months before the shear pin could be reinstalled (after removing a rock from inside the scraped surface unit!). After such a long time, it was remarkable that simply replacing the shear pin was sufficient to reactivate the sprocket and have it work properly.
Shear pin sprockets also wear out due to wear between the teeth and the drive chain. Armstrong shear pin sprockets thus may be used as replacement items when the older type wear out. These sprockets have been used frequently as replacement parts on scraped surface exchangers and chillers originally made by other firms.
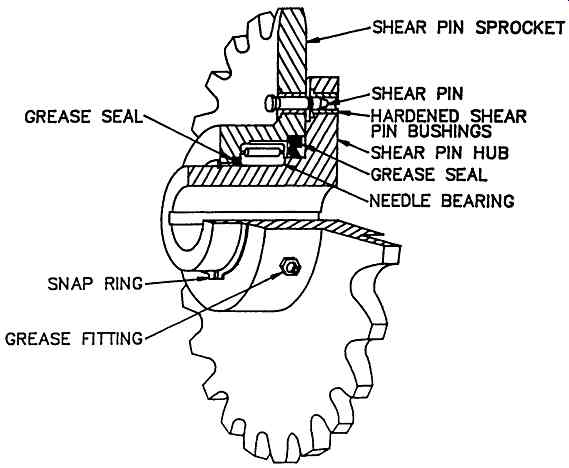
FIG. 9 Schematic drawing of shear pin sprocket. (Armstrong Engineering Associates,
Inc West Chester, PA) Components: SHEAR PIN SPROCKET; GREASE; SHEAR PIN;
HARDENED SHEAR PIN BUSHINGS; SHEAR PIN HUB GREASE SEAL; NEEDLE BEARING; SNAP
RING; GREASE FITTING