AMAZON multi-meters discounts AMAZON oscilloscope discounts
WITH WATER, ONE OF THE DESIGNER'S first concerns is to match the quality of the water to the task it performs. As this becomes a more serious design issue, designers will provide for the recycling of water within and around buildings, as well as specify plumbing fixtures that use less water.
Table 1 shows typical relationships between water quality and usage. Water recycling and conservation are discussed in detail in Section 22. In this section we deal primarily with potable (drink able) water: first with issues of water quality and then with the matter of ensuring an adequate supply of water throughout a building.
1. WATER QUALITY
A summary of water quality at the various stages of the hydrologic cycle was shown in FIG. 4. As precipitation, water contains few impurities: almost no bacterial content is present, and only small amounts of minerals and gases can be expected. Surfaces are needed to collect this nearly pure water-and from these surfaces, foreign substances can readily contaminate the water. These pollutants can affect water's physical (mostly organic), chemical (mostly inorganic), biological, or radiological characteristics. Both surface water and groundwater are subject to pollution.
The U.S. National Drinking Water Clearing house is a source of information on water sup ply and wastewater treatment systems. Its brief history of waterborne diseases worldwide begins with a major cholera epidemic that began in Calcutta, India, in 1817, killing thousands and spreading by 1832 to New York City, emptying the streets in panic over the disease. In 1854, a London physician demonstrated that local cases of cholera could be traced to one pump contaminated with sewage from a nearby house, but how the disease was transmitted remained a mystery. In summer 1859, London's Thames River, carrying combined storm and sanitary wastes, stank so badly that Parliament was suspended. In 1892, the bacterium causing cholera was identified during an epidemic in Hamburg, Germany, proving the relationship between contaminated water and the disease. In 1939, an outbreak of typhoid fever killed 60 people at an Illinois mental hospital; typhoid and gastroenteritis resulted from an accidental pumping of polluted river water into supply mains in Rochester, New York, in 1940. Another worldwide cholera epidemic began in Indonesia in 1961, eventually reaching Latin America by 1991. In 1993, an out break of cryptosporidiosis in the public water supply of Milwaukee, Wisconsin, shook the public's faith in water systems across the United States; that out break killed 104 and infected more than 400,000 people.
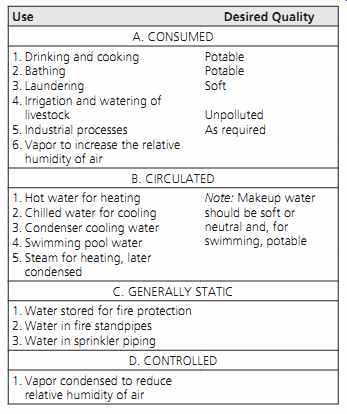
Table 1 Water Use and Quality in Buildings
In response to this history, we disinfect huge quantities of water, expending energy and adding chemicals so that every drop of water entering North American buildings is potable. Again, the high-grade resource/low-grade use question arises; recycled water for lower-grade use is discussed in Section 22.
(a) Physical Characteristics
Some of the most noticeable aspects of water quality fall within this category. Water from surface sources (roof runoff, streams, rivers, lakes, ponds) is particularly subject to physical pollutants.
Turbidity is easy to see and thus is a likely source of dissatisfaction for the would-be consumer. It is caused by the presence of suspended material such as clay, silt, other inorganic material, plankton, or finely divided organic material. Even those materials that do not adversely affect health are usually aesthetically objectionable.
Color, another visible alteration, is often caused by dissolved organic matter, as from decaying vegetation. Some inorganic materials also color water, as do microorganisms. Like turbidity, such color changes usually do not threaten health, but they are often psychologically undesirable.
Taste and odor can be caused by organic com pounds, inorganic salts, or dissolved gases. This condition can be treated only after a chemical analysis has identified which source is responsible.
Temperature is another characteristic of psychological importance-we expect drinking water to be cool. In general, water supplied between 50º and 60ºF (10º and 16ºC) is preferred.
Foamability is usually caused by concentrations of detergents. The foam itself does not pose a serious health threat, but it may indicate that other, more dangerous pollutants associated with domes tic waste are also present. Because of increased foaming in water in the 1960s, today's detergents must use linear alkylate sulfonate (LAS), which bio degrades rapidly-except in the absence of oxygen.
Because this lack of oxygen is characteristic of some septic tank drainage fields, foam in drinking water should be investigated promptly.
(b) Chemical Characteristics
Groundwater is particularly subject to chemical alteration because as it moves downward from the surface it slowly dissolves some minerals contained in rocks and soils. A chemical analysis (such as that given in Table 2) is usually required for individual water supply sources. Such analysis will indicate (1) the possible presence of harmful or objectionable substances, (2) the potential for corrosion within the water supply system, and (3) the tendency for the water to stain fixtures and clothing. Concentrations are expressed in milligrams per liter (mg/L), which is essentially equivalent to parts per million (ppm).
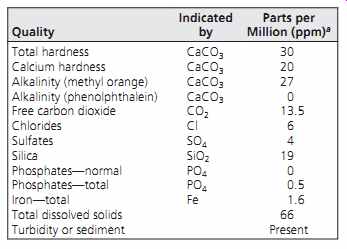
Table 2 Example of Chemical Analysis of Water
Some general terms commonly used to describe chemical characteristics of water are as follows:
Alkalinity. Caused by bicarbonate, carbon ate, or hydroxide components. Testing for these components of water's alkalinity is a key to determining which treatments to use.
Hardness. A relative term (see FIG. 1). Hard water inhibits the cleaning action of soaps and detergents, and it deposits scale on the inside of hot water pipes and cooking utensils, thus wasting heating fuel and making utensils unusable. Hardness, which is caused by calcium and magnesium salts, can be classified as temporary (carbonate) or permanent (non-carbonate). Temporary hardness is largely removed when the water is heated-it forms the scale just described. Permanent hardness cannot be removed by simple heating (see Section 4c).
pH. A measure of the water's hydrogen ion concentration, as well as its relative acidity or alkalinity (FIG. 1). A pH of 7 is neutral. Measurements below 7 indicate increasing acidity (and corrosiveness); water in its natural state can have a pH as low as 5.5, with 0 being the ultimate acidity. Measurements higher than 7 indicate increasing alkalinity. A pH as high as 9 can be found in water in its natural state, with 14 representing the ultimate alkalinity.
The pH value is the starting point for determining treatments for corrosion control, chemical dosages, and disinfection.
Unintentional chemical additions to water supplies most commonly include the following elements:
Toxic substances are occasionally present in water supplies. Local health authorities can pro vide information about acceptable concentrations of such substances as arsenic (As), barium (Ba), cadmium (Cd), chromium (Cr6+), cyanide (CN), fluoride (F), lead (Pb), selenium (Se), and silver (Ag). Although limited amounts of fluoride are frequently added to water supplies to help prevent tooth decay, fluorides in excess of such optimum concentrations can produce mottling of teeth. Lead poses a dangerous threat, even in relatively small amounts, because it is a cumulative poison. Lead in water usually comes from lead piping (in older buildings or cities) or from corrosive water on lead-painted roofs. The maximum recommended concentration is 0.05 mg/L.
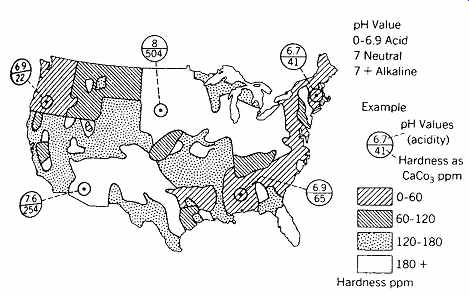
FIG. 1 Approximate groundwater chemical characteristics across the United
States. Treatment may be needed when the pH is less than 7.0 (acidity results
in corrosion) or when hardness as CaCO3 exceeds 65 ppm (ppm and mg/L are essentially
identical measures). (Courtesy of Progressive Architecture.)
Unfortunately, the list of toxic chemicals is expanding, and as chemical waste dumps have been abandoned or mismanaged, groundwater has become contaminated. The U.S. Environmental Protection Agency (EPA) has estimated that 75% of both active and abandoned chemical waste dumps-some 51,000 in all-are leaking. In addition to the following list of inorganic chemicals, we are becoming aware of many new organic chemicals as well, some of which are suspected of causing 5% to 20% of U.S. cancers. The threat to our groundwater supplies is illustrated in FIG. 2.
Once polluted, aquifers are extremely difficult to clean. This is one reason why ground source heat pumps are tightly regulated.
Chlorides can enter water as it passes through geologic deposits formed by marine sediment, or because of pollution from seawater, brine, or industrial or domestic wastes. A noticeable taste results from chloride in excess of 250 mg/L.
Copper can enter water from natural copper deposits or from copper piping that contains corrosive water. Concentrations of copper in excess of 1.0 mg/L can produce an undesirable taste.
Iron is frequently present in groundwater. Corrosive water in iron pipes will also add iron to water.
At concentrations above 0.3 mg/L, iron can lend a brownish color to washed clothes and can affect the taste of the water.
Manganese can both pose a physiological threat (it is a natural laxative) and produce color and taste effects similar to those produced by iron. The recommended limit is 0.05 mg/L.
Nitrates in high concentrations pose a threat to infants, in whom they can cause "blue baby" disease. In shallow wells, nitrate concentrations can indicate seepage from deposits of livestock manure.
Pesticides, a growing threat to water supplies, are particularly common in wells near homes that have been treated for termite control. Avoid using pesticides near wells.
Sodium is primarily dangerous for people with heart, kidney, or circulatory ailments. For a low sodium diet, the sodium in drinking water should not exceed 20 mg/L. Salts spread on roadways for ice control can leach into the soil and enter ground water. Note that some water softeners (discussed in Section 4c) can raise sodium concentrations in water.
Sulfates, which have laxative effects, can enter groundwater from natural deposits of Epsom salts (magnesium sulfate) or Glauber's salt (sodium sulfate). Concentrations should not exceed 250 mg/L.
Zinc sometimes enters groundwater in areas where it is found in abundance. Although not a health threat, it can cause an undesirable taste at concentrations above 5 mg/L.
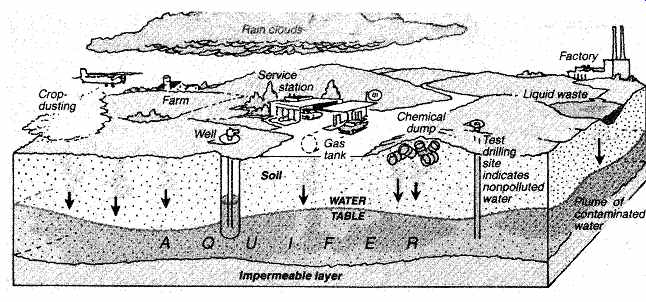
FIG. 2 How groundwater becomes contaminated. The "plume" formed
by contaminants can often go undetected. (© 1982 by Newsweek, Inc.; all rights
reserved. Reprinted by permission.)
(c) Biological Characteristics
Potable water should be kept as free as possible of disease-producing organisms-bacteria, protozoa, and viruses. These organisms are not easily identified; a thorough biological water test is complex and time-consuming. For this reason, the standard test is for one kind of bacteria-the coliform group (Escherichia coli, better known as E. coli), which is always present in the fecal wastes of humans (as well as those of many animals and birds) and which outnumbers all other disease-producing organisms in water. The recommended maximum concentration of coliform bacteria is one organism per 100 mL (about ½ cup) water.
For biological activity to be kept to a minimum in drinking water, a water source should be chosen that does not normally support much plant or animal life, hence the popularity of groundwater rather than surface water as a source. In addition, the supply should be protected from subsequent biological contamination. Where cities depend on small lakes for water, human beings are frequently excluded from the watersheds. Organic fertilizers and nutrient minerals should also be kept out of the water supply to further discourage biological activity. For the same reason, stored water should be kept dark and at low temperatures. Finally, organ isms (or their by-products) are commonly destroyed at treatment facilities.
(d) Radiological Characteristics
The mining of radioactive materials and the use of such materials in industry and power plants have produced radiological pollution in some water supplies. Because radiological effects are cumulative, concentrations of radioactive materials should be low indeed. "Safe" minimum concentrations have continually been revised downward for other radiation exposures; consult the local public health service for current recommendations.
Table 3 Common Water Quality Problems and Treatment in Small Systems
2. FILTRATION
In the preceding section, water pollution was broken down into physical, chemical, biological, and radio logical categories. The various forms of treatment for such pollutants do not necessarily fall into the same categories, as one treatment may be effective for several different polluted conditions. A general look at common domestic water quality problems and treatments is provided in Table 3.
We begin with filtration, because so often it is the first treatment in a series; it is also one of the oldest and simplest methods. This very common treatment removes suspended particles, some bacteria, and color or taste by passing water through a permeable fabric or a porous bed of materials. The more common approaches are listed in the following subsections, beginning with filtering to remove suspended particles and then moving on to more specialized applications for the removal of iron and/or manganese, tastes, and odors.
(a) Sedimentation
Before water enters a filter, this process removes some suspended matter simply by allowing time, the inactivity of the water, and gravity to do the work of settling out heavier suspended particles.
Simple basins, ponds, or tanks constructed for this purpose are large enough to retain the water for at least 24 hours and are equipped with baffles to slow the water flow. To clean out the sediment, water usually is diverted to an identical second basin while the first is being cleaned.
(b) Coagulation
This process also removes suspended matter, along with some coloration. A chemical such as alum (hydrated aluminum sulfate) is added to water made turbulent by baffles or static mixers to distribute the chemicals evenly.
(c) Flocculation
The water is then held in a quiet condition in which the suspended particles will combine with the alum to form floc. These heavy particles then settle out in a process similar to sedimentation. Some adjustment of the pH may be necessary.
See the National Drinking Water Clearinghouse, Tech Brief (September 1996), Filtration, for details on the following methods of filtering.
(d) Slow Sand Filters
These are common in small-scale water supply systems (FIG. 3; also see FIG. 8 for a rainwater application). Not suitable for water with high turbidity, they do not usually require coagulation/flocculation and may not even require sedimentation.
Water should not be chlorinated before entering this filter, because it will interfere with the subsequent biological activity. These filters are able to remove up to 99.9% of Giardiacysts.
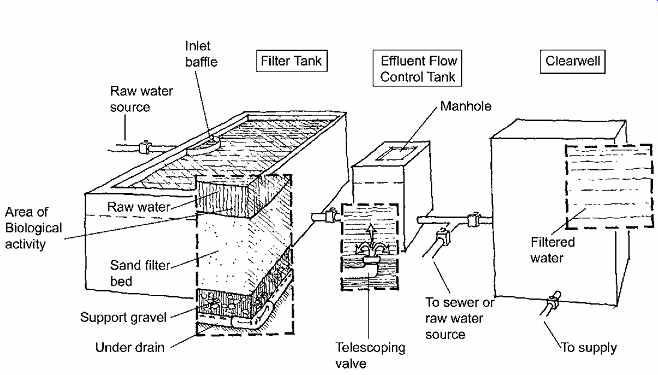
FIG. 3 Slow sand filter effective in removing cysts of Giardia. Water should
not be chlorinated before it enters the filter. (From National Drinking Water
Clearinghouse. Tech Brief [September 1996], Filtration. U.S. Environmental
Protection Agency. Washington, DC. Redrawn by Dain Carlson.)
Slow sand filters are low-maintenance, easily constructed devices that should be cleaned as often as the turbidity of the water demands-from once a day to perhaps once a month. They are cleaned by removal and replacement of about the top 1 in. (25 mm) of sand, which has formed a layer of biological slime called (descriptively) the schmutzdecke, which traps small particles and degrades organic material in the water. This sand is then either washed for reuse or discarded.
The approximate rate of flow is slow, requiring a rather large surface area: 0.03 to 0.10 gpm per ft 2 (0.02 to 0.07 L/s m2) of filter bed surface; in other units, 40 to 140 gallons per day per square foot (1630 to 5720 liters per day per square meter) of filter bed surface. Overall thickness is usually 30 to 48 in. of sand over 12 in. of gravel (900 to 1200 mm of sand over 300 mm of gravel) with an underdrain system.
In freezing temperatures, slow sand filters must be housed; if they develop an ice layer, this prevents cleaning.
(e) Diatomaceous Earth Filters Also known as precoat or diatomite filters, these can be of either the vacuum or the pressure type. They rely on a layer of diatomaceous earth, a minimum of 1/8 in. (3 mm) thick placed on a septum or filter element (for Giardia removal, the thickness should be increased to about 1/5 in. [5 mm]). They are most suitable for water with low bacterial counts and low turbidity. Simple to operate and effective in removing cysts, algae, and asbestos, they require periodic attention to remain effective, including backwashing every 1 to 4 days.
(f) Direct Filtration
Intended for water supplies of high quality and seasonally consistent flow, these systems omit sedimentation but should include coagulation for most effective Giardia removal. These are often used with steel pressure tanks to maintain pressure in the water supply line.
Packaged filtration combines features such as chemical addition, flocculation, and sedimentation, along with filtration, in one compact unit. Most often used for small community water supplies, these systems treat surface water to remove turbidity, color, and coliform organisms.
(g) Membrane Filtration
Also called microfiltration or ultra-filtration, this rapidly developing technique can remove bacteria, Giardia, and some viruses. It does not require coagulation as pretreatment. Using hollow fiber or spiral wrapped membranes, it is able to exclude all particles greater than 0.2 micron from the water stream. It is best used on water supplies of low turbidity because of fouling of the fibers or membranes.
Water is forced at high pressure through these filters. The contaminants trapped on the inflow side must be frequently removed by reversing the flow and flushing the waste; calcium and other persistent contaminants must be periodically removed with chemical cleaning.
Nanofiltration, using much smaller pores, is discussed in Section 3.
(h) Cartridge Filtration
Increasingly popular on lavatory faucets as well as on small supply systems, these systems are easy to operate and maintain. They require water of low turbidity and last longer when some pre-filtering by more crude means is performed upstream. They can exclude particles of 0.2 micron (or even smaller). A disinfectant can prevent surface-fouling microbial growth on the cartridge filters; some periodic chemical cleaning will likely be required.
(i) Other Filters
Activated carbon filters are particularly effective for removing tastes and odors. The water is passed through granular carbon, which attracts large quantities of dissolved gases, soluble organics, and fine solids.
Porous stone, ceramic, or unglazed porcelain filters (also called Pasteur filters) are usually made in small sizes so that they can be attached to water faucets.
They are used widely in some countries, such as Mexico, but poor maintenance or hairline cracks often lead to bacterial infiltration, complicating the filtration process. A more positive approach to the disinfection of drinking water thus is desirable.
3. DISINFECTION
Disinfection is the most important health-related water treatment, because it destroys microorganisms that can cause disease in humans. Disinfection is required of water supply systems that rely on surface water or groundwater sources under the influence of surface water. Initially, primary disinfection achieves the desired level of microorganism kill (inactivation); then secondary disinfection maintains a disinfectant residual in the treated water that prevents microorganism regrowth.
Although chlorination has become the standard approach to removing harmful organisms from water, there are alternatives: nanofiltration, ultraviolet (UV) radiation (unsuitable for water with high turbidity because it cannot easily penetrate), bromine, iodine, ozone, and heat treatment, among others. Chlorine continues to disinfect after the initial application. It is this continuing secondary disinfection that has made it universally relied on, despite dangers such as that posed by deadly chlorine gas. Although chlorine affects the taste and odor of water, it is also effective in removing less desirable tastes and odors. Unfortunately, chlorine can react with organic materials in water to form halogenated by-products. It is easier to either remove the organic materials before treatment, or to use another disinfectant strategy, than to try to remove these halogenated by-products after chlorine treatment.
See the National Drinking Water Clearing house, Tech Brief (June 1996), Disinfection, for details on the methods presented in Subsections 3(a) through 3(d).
(a) Chlorination Factors that affect chlorine's ability to disinfect include:
1. Chlorine concentration. The higher the concentration, the faster and more complete the rate of disinfection.
2. Contact time. The longer the chlorine contacts the organisms in water, the more complete the disinfection. At a minimum, 0.4 mg/L of chlorine should contact water 30 minutes before use.
3. Water temperature. The higher the temperature during contact, the more complete the disinfection.
4. pH. The lower the pH, the more effective the disinfection.
There are three common forms in which chlorine is used to disinfect water supplies. Chlorine gas is stored in a cylinder (FIG. 4) as a liquid under high pressure and is released (as a gas) by a regulator to an injector attached to a water pipe or tank. The injector passes highly pressurized water through a venturi orifice, creating a vacuum that draws the chlorine into the water stream.
Sodium hypochlorite solution is easier to handle than deadly chlorine gas but is very corrosive and decomposes rather quickly. It should be stored in a cool, dark, dry area for no more than a month.
Hypochlorinators automatically pump (or inject) a sodium hypochlorite solution into water. They are usually no larger than the pumps used in small water systems. Some hypochlorinators are specially designed for low and fluctuating water pressures or for use where electricity is not available.
Solid calcium hypochlorite is a white solid containing 65% available chlorine that dissolves easily in water. It is corrosive, with a strong odor, but very stable and can be stored for up to a year. However, it readily absorbs moisture, forming chlorine gas; also, reactions between calcium hypochlorite and organic materials (wood, cloth, petroleum products) can generate enough heat to cause a fire or explosion. Again, hypochlorinators are used to deliver the disinfectant to water.
(b) Chloramine
This is generated on site by adding ammonia to water containing chlorine or when water containing ammonia is chlorinated. This is a weaker disinfectant against viruses or protozoa than the chlorination processes, but it produces fewer disinfection by-products. It is most often used as a secondary rather than a primary disinfectant. Again, hypochlorinators are used to inject chlorine, after which ammonia is added.
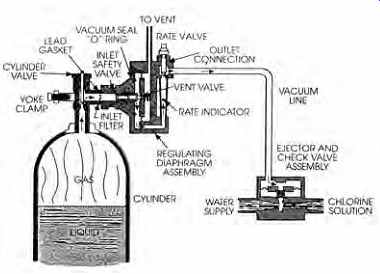
FIG. 4 Cylinder-mounted chlorinator for automatic disinfection of supply
water. (From National Drinking Water Clearinghouse. Tech Brief [June 1996],
Disinfection. U.S. Environmental Protection Agency. Washington, DC.)
(c) Ozonation
This was first used in full-scale drinking water treatment in 1906. It is a powerful oxidizing and disinfecting agent, destroying most bacteria, viruses, and other pathogenic organisms. It requires a shorter contact time and dosage than chlorine and leaves no chlorine taste. Ozone is formed by passing dry air (or pure oxygen) through a system of high voltage electrodes. It is an unstable gas and must be generated on site. When ozone reacts with an organic, it produces oxygen and an oxidized form of the organic. Ozone not used in this process quickly decays to oxygen.
In the United States, ozone is commonly used in cooling tower water treatment (FIG. 5), to effectively deal with Legionella pneumophila and control algae and scale growth that can greatly reduce cooling efficiency. Ozone is also used in food processing, waste water cleanup, smoke removal, swimming pools and spas, bottled water, and pulp and paper bleaching.
Equipment includes an ozone generator, a contactor, and a destruction unit, plus instrumentation and controls. Operation and maintenance are relatively complex; electricity accounts for 26% to 43% of the operating costs for small systems. Because it acts only as a primary disinfectant, a secondary disinfectant (often chlorine) is usually required.
(d) Ultraviolet Radiation
Special lamps are used within a reactor (FIG. 6), whose radiation disrupts the genetic material of the cells of organisms, making them unable to reproduce. Although effective against bacteria and viruses, UV radiation does not inactivate either Giardia or Cryptosporidium cysts. Otherwise, it is an effective primary disinfectant system, requiring a short contact time and without halogenated by-products.
Yet again, a secondary disinfectant system is usually necessary. This system is not suitable for water that contains high levels of suspended solids, turbidity, color, or soluble organic matter.
(e) Nanofiltration
These filter membranes start with pore sizes of 0.2 to 0.3 micron and are then dipped into a polymer that leaves a thin film, decreasing the pore size to 1 nanometer. This pore size removes bacteria, viruses, pesticides, and organic material. It also gives the membranes an affinity for calcium, contributing to water softening. However, it also means that the membranes need periodic acid cleaning to remove the calcium deposits. Adding phosphates to nano-filtered water reduces its capacity to dissolve lead.
With such extremely small pore sizes, this pro cess requires very high water pressures, in turn requiring energy. Yet again, a secondary disinfectant system is usually necessary.
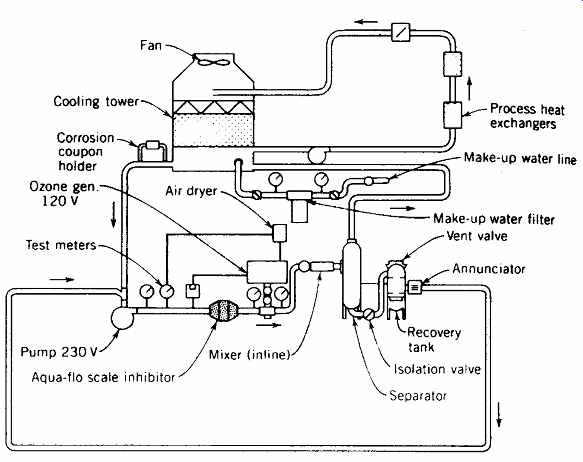
FIG. 5 Recycled cooling tower water is treated by an ozonator, a magnetic
de-scaler, and a filtration system. This controls scale formation, algae and
slime, corrosion, and sludge buildup. (Courtesy of Aqua-Flo, Inc., Baltimore,
MD.)
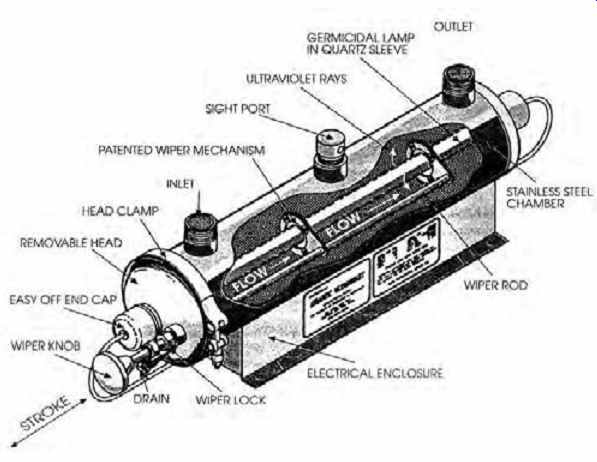
FIG. 6 Ultraviolet water purifier, effective against bacteria and viruses
(but not against Giardia or Cryptosporidium cysts). (Courtesy of Atlantic Ultraviolet
Corporation, Hauppauge, NY.)
4. OTHER WATER TREATMENTS
(a) Aeration (Oxidation)
This simple process can improve the taste and color of water and help remove iron and manganese. In aeration, as much of the water's surface as possible is exposed to air. The methods used are rich in aesthetic possibilities-the spraying of water into air, the fall of a turbulent stream of water over a spill way, and flow-forms, sculptural waterfalls designed to carry water in a rhythmical, pulsating, figure eight pattern. The Real Goods headquarters in Hopland, California, features some of these water features (FIG. 7) and water recycling.
To guard against contamination, these processes are often enclosed; if exposed, they must be kept clean. For aeration within tanks, water is passed through a series of perforated plates in streams or droplets.
Aeration improves the flat taste of distilled water and cistern water by adding oxygen. It also oxidizes iron or manganese, which then can more easily be removed by filtration. It also removes odors caused by hydrogen sulfide and algae.
Because aeration raises the level of dissolved oxygen in water, it should be avoided as a treatment when corrosion is a threat.


FIG. 7 The Real Goods Solar Living Center at Hopland, California, illustrates
photovoltaic (PV) power, passive solar heating, daylighting, and passive cooling,
as well as creative water recycling. Water from on-site irrigation ponds (a)
is pumped by PV to a tank on a small hill, and (b) water is also dispersed
into the air from the Agave Fountain for evaporative cooling. (Landscape design
by Land and Place.) (Photos © 2009 Alison Kwok, all rights reserved.)
(b) Corrosion Control
It is important to control corrosion both to keep water systems operating freely and to prevent corrosive water from increasing the concentration of hazardous materials (as from copper pipes). Corrosion also imparts a taste and/or odor to water that is objectionable. Corrosion is a slow degradation of a metal by a flow of electric current from the metal to its surroundings. Some factors involved in corrosion control are:
1. Acidity. The more acid (low pH, less than 6.0), the more corrosive the water.
2. Conductivity. As dissolved mineral salts increase the water's conductivity, they encourage the flow of the electrical current of corrosion.
3. Oxygen content. Dissolved oxygen destroys the thin protective hydrogen film on immersed metals, thus promoting corrosion.
4. Carbon dioxide content. Carbon dioxide forms carbonic acid, which attacks metal surfaces.
5. Water temperature. Increased temperature increases corrosion.
6. Lower flow rates. Reduced turbulence means reduced erosion of the protective layers that form on the inner surfaces of pipes.
The products of corrosion often contribute to scale formation. Scale then lines surfaces, eventually clogging openings.
See the National Drinking Water Clearing house, Tech Brief (February 1997), Corrosion Control, for details on this subject.
Acid neutralizers can be installed on water sup plies with low pH; their function is often combined with those of hypochlorinators. Typically, neutralizing solutions are mixtures of lime, soda ash, and water. However, pH adjustment should be made just before water delivery, after treatment processes such as coagulation and disinfection.
Corrosion inhibitors cause protective coatings to form on pipes. They are commonly fed into the water, as are other chemicals. Inorganic phosphates, sodium silicates, and mixtures of phosphates and silicates are the more commonly used additives.
Other corrosion control strategies include commercial pipe coatings/linings, installing dielectric or insulating unions (to avoid complications from dissimilar pipe metals), and avoiding metal piping and fixtures altogether.
(c) Softening
Water hardness is caused primarily by calcium and magnesium deposits; when they are removed, water will be soft. Where water hardness is mild enough to affect only laundering, cisterns may be used to collect soft rainwater to use for washing clothes. Where water hardness produces scale in pipes and water heating appliances, and cisterns are not feasible, water-softening equipment is used. Demineralization of water is accomplished with one of three methods: ion exchange, reverse osmosis, or electrodialysis.
See the National Drinking Water Clearing house, Tech Brief (May 1997), Ion Exchange and Demineralization, for details on the following methods of treatment.
Ion exchange is popular for small systems, and is effective not only with hardness ions but also with radionuclides. It can be used with fluctuating flow rates but requires pretreatment of most surface waters, and its waste is highly concentrated (requiring careful disposal). A large variety of resins are used for the exchange medium, each effective for specific contaminants.
On the exchange medium's charged surface, one (contaminant) ion is exchanged for another (regenerant) ion. Eventually, saturation occurs; the contaminants are flushed and the medium is regenerated-once per day is the common shortest cycle. Sodium chloride is often used to regenerate the exchange medium, resulting in a rather high sodium residual-an undesirable development for people on low-sodium diets. Another regenerant material is potassium chloride.
Equipment includes prefiltration, ion exchange, disinfection, storage, and distribution elements; in smaller systems, single-package units incorporate all of these processes.
Reverse osmosis (RO), like ion exchange, is popular for small systems and can be used with fluctuating flow rates, but it requires pretreatment of most surface waters, and its waste is also highly concentrated. RO is effective not only with hardness ions but also with radium, natural organic substances, pesticides, and microbiological contaminants. RO units used in series can remove an even higher percentage of contaminants.
An inert, semipermeable membrane has a high-pressure supply water on one side; as the pres sure slowly forces water through this filtering membrane, most of the contaminants are removed. Water must be used to flush the membrane so that mineral buildup is avoided; this produces a brine requiring careful disposal. Membranes are available in varying types and pore sizes; they are prone to fouling.
Commercial RO units are available in sizes ranging from a 1 gpm (3.9 L/m) water delivery rate (using two membranes and a 3-hp [2.2-kW] motor, requiring 4.5 gpm [17 L/m] feedwater for a 22% recovery rate) to a water delivery rate of 12.5 gpm (47.3 L/m) (using 12 membranes and a 15-hp [11.2-kW] motor, requiring 19.2 gpm [72.8 L/m] for a 65% recovery rate).
Electrodialysis effectively removes fluoride and nitrate, and can also remove barium, cadmium, and selenium. It is relatively insensitive to levels of flow and of total dissolved solids but has an enormous appetite for water. From 10% to 80% of the total water supplied to an RO filtering unit is delivered to the user; the remainder is a waste stream. It also requires a higher level of pretreatment.
In this process, membranes adjacent to the inflowing stream are charged (either positively or negatively), attracting counter-ions to these membranes. The membranes allow either positively or negatively charged ions to pass through; thus, the ions leave the inflow stream and enter the waste streams (on the other side of each membrane). High water pressure and a source of dc power are needed in this process.
Membranes can become fouled when the pores are clogged by salt precipitation or blocked by suspended particulates. For either of these water conditions, pretreatment is essential. Reversing the charge on the membranes, electrodialysis reversal, helps to flush the attached ions from the membrane surface and can extend the time between membrane cleanings.
(d) Nuisance Control
Some organisms may not be injurious to health but can multiply so rapidly that piping or filters become clogged, or the water's appearance, odor, and taste are affected. This situation is most common with surface water sources, and it is within surface reservoirs that these treatments are most often applied.
Algae growths, the most prevalent nuisance, can usually be controlled by applying copper sulfate (blue stone or blue vitriol) to the water body. Sudden and massive algae kills can have adverse impacts on other life forms within the water, because the decomposing algae rob the water of oxygen. As a further precaution, stored water should be shielded from sunlight whenever possible.
Cooling towers present an especially difficult water treatment problem. The murky water with high turbidity and a high bacterial count leads to clogged passages (thus inefficient performance), deteriorated surfaces, and the growth of potentially lethal bacteria (L. pneumophila). As a result, enormous quantities of water are commonly passed once-through a cooling tower, and then dumped into storm sewers rather than being recycled in a closed system. (As late as 1989, San Francisco's City Hall was reported to be using 96,000 gallons [363,000 L] per summer day for once-through cooling-in a year of drought.) To treat cooling tower water successfully, a method is needed for microbial control, removing organics, and precipitating inorganics. Ozonation (FIG. 5) is a common answer to this widespread treatment problem.
(e) Fluoridation
A heated controversy continues over the addition of fluoride to drinking water. The advantage of fluoridation is that children who drink fluoridated water during the most active stages of tooth development have lower rates of tooth decay. Because everyone drinks water, all children (and adults) benefit, not just those who can afford fluoride pills or prescription toothpaste. Opponents of fluoridation suggest that because sugar is a leading cause of tooth decay in children, sugar, rather than water, should be fluoridated. Small water systems can be equipped with fluoridation units. However, fluoride levels in the water supply must be care fully monitored. In amounts above those used in water treatment, fluoride is toxic and can cause mottled teeth.
(f) Distillation
This is a simple, low-technology approach to purification that produces the equivalent of bottled water for drinking, cooking, and laboratory uses. In one process, it promises the removal of suspended sol ids, salts, bacteria, and (apparently) halogenated hydrocarbons. When water pollution is extreme, as in the case of sea (salt) water, distillation may be the best treatment. Water is heated to encourage evaporation. As the water turns to vapor, virtually all pollutants are left behind. When this vapor encounters a cooler surface, it condenses, and pure water (although flat in taste) can be collected from this surface.
Any heat source can be used in the distilling of water; solar stills (FIG. 8) are gaining in popularity because the energy used is free. Solar distillation of cistern water is an autonomous approach. In semi arid, rather sunny climates, a solar still should pro duce about ½ gal per square foot (4 L/m2) of collector surface area per day. This rate of production suggests that only the water used directly for drinking or other specialized purposes is usually feasible for distillation. Another factor to consider is that the cleaning of the still is generally accomplished by flushing it with twice as much water as was delivered. If not excessively brackish, this flush water could be used for irrigation or other non-potable-quality tasks.

FIG. 8 A solar still can be used to provide a small daily quantity of pure
water; this installation serves a laboratory. (Courtesy of McCracken Solar
Co., Alturas, CA.)