AMAZON multi-meters discounts AMAZON oscilloscope discounts
(cont. from part 1)
8. Colloidal Membrane Foulants
8.1 Description
Colloidal foulants are inorganic and organic compounds that naturally exist in suspension and may be concentrated by the RO separation process and precipitate on the membrane surface, thereby causing membrane flux to decline over time. Colloidal solids have a particle size of 0.001 to 1 µm. For prevention of colloidal fouling, RO membrane manufacturers usually recommend a feed turbidity of less than 0.1 NTU and an SDI15 of less than 4.
Metal oxide and hydroxide foulants most frequently encountered during brackish and seawater desalination are iron, manganese, copper, zinc, and aluminum. Typically, open ocean seawater contains very low levels of these metal foulants, and there fore, if such fouling is encountered on the membrane elements, the usual sources are overdosing of coagulant (iron salt) or corrosion of pipes, fittings, tanks, and other metal equipment located upstream of the SWRO system.
Iron and Manganese
Iron and manganese fouling may occur if source water is collected via subsurface intake from a brackish aquifer with a naturally high content of these metals or from a coastal aquifer which is under the influence of fresh groundwater that contains high levels of these compounds in reduced form (iron of more than 2 mg/L as ferrous or manganese of more than 0.5 mg/L).
If iron and manganese are in reduced form and they are below 1.0 and 0.1 mg/L, respectively, they can be removed by RO membranes without causing accelerated fouling. However, if iron and manganese are in oxidized form, their levels should be reduced below 0.05 and 0.02 mg/L, respectively, to prevent mineral fouling of the membranes.
In addition to naturally occurring colloidal matter, colloidal iron fouling on the surface of RO membranes may be caused by corrosion of upstream piping and equipment or by overdosing or poor mixing of iron-based coagulant used for conditioning the source water. If the source water contains chlorine, this colloidal iron tends to catalyze the oxidation process caused by chlorine, which in turn enhances the damage to the RO membranes even when residual chlorine in the saline source water is in very small doses.
Silica Another mineral fouling compound frequently encountered in brackish aquifers is silica. Total silica (silicon dioxide) in the source water consists of reactive silica, which is in soluble form, and unreactive silica, which is in colloidal form. While reactive silica is not a challenge for RO membranes, colloidal silica in the saline source water can cause significant membrane fouling. It should be pointed out, however, that elevated content of silica in colloidal form is mainly found in brackish water sources; unreactive silica is present in very low levels in seawater and is fouling challenge only when its level in the concentrate exceeds 100 mg/L.
The stability of colloids is reduced with an increase in source water salinity, and therefore typical seawater with a TDS concentration in a range of 30,000 to 45,000 mg/L would contain silica in dissolved and precipitated forms rather than in colloidal form.
Open ocean seawater typically contains silica of less than 20 mg/L, and therefore this compound does not cause mineral fouling of SWRO membranes.
However, if the source seawater is collected via a subsurface well intake which is under the influence of a brackish coastal aquifer with a high content of colloidal silica, or it is collected near an area where a silt-laden river enters into the ocean, then colloidal fouling may become a challenge. Colloidal foulants can be removed by coagulation, flocculation, sedimentation and filtration, similar to particulate foulants.
Polymers
Another common source of colloidal fouling is overdosing or poor mixing of polymers used for conditioning of the saline source water prior to filtration. Such a problem usually occurs when the source water contains fine particulates of relatively low quantity and electric charge (i.e., the water is of very low turbidity, typically below 0.5 NTU) and a polymer is added to enhance the flocculation of such particles.
Hydrocarbons
The most common organic colloidal foulants are oil-product-based hydrocarbons. Such compounds are not contained naturally in most brackish waters or in open ocean seawater, and their occurrence indicates that the saline water intake area or aquifer is under influence of man-made sources of contamination--typically discharge from a wastewater treatment plant, from storm drains collecting surface runoff from urban areas (parking lots, industrial sites, etc.), or from waste discharges or oil leaks released by ships, boats, or near-shore oil storage tanks in port areas.
Even in very small quantities (0.02 mg/L or more), oil and grease can cause accelerated fouling of RO membranes. Therefore, it is desirable that oil and grease content in the source water be maintained below 0.02 mg/L at all times.
8.2 Parameters and Measurement Methods
Colloidal Iron, Manganese, and Silica
Colloidal iron, manganese, and silica are measured by standard laboratory tests. Usually colloidal (also referred to as unreactive) silica is determined by measuring total and reactive silica in the source water.
Total Hydrocarbons
Total hydrocarbon concentration can be measured by both laboratory analytical methods and online analyzers. Most desalination plants using surface water sources (i.e., a saline river estuary or ocean water) are equipped with online total hydrocarbon analyzers.
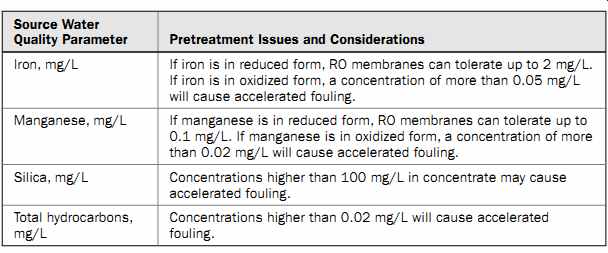
Table 5 Water Quality Parameters for Characterization of Colloidal Foulants
8.3 Threshold Levels of Colloidal Foulants
Table 5 presents key source water quality parameters which operators are recommended to measure in order to characterize potential colloidal foulants in the source water.
9. Mineral Membrane-Scaling Foulants
9.1 Description
All minerals contained in the source water are concentrated during the process of membrane salt separation. As their concentration increases during the desalination process, ions of calcium, magnesium, barium, strontium, sulfate, and carbonate can form sparingly insoluble salts, which could precipitate on the RO membrane surface. The mineral scales that typically form during desalination are those of calcium carbonate, calcium and magnesium sulfate, and barium and strontium sulfate.
Formation of mineral scales on the membrane surface is balanced by the high salinity of the source water, which tends to increase the solubility of all salts. This means that the higher the salinity of the source seawater, the less likely a mineral scale is to form on the membrane surface at typical seawater pH of 7.6 to 8.3 and desalination system recovery of 45 to 50 percent. In brackish seawater desalination systems, which typically operate at much higher recoveries (75 to 85 percent) and use source waters that have relatively lower ionic strength, mineral scaling is a frequent problem.
In typical seawater desalination systems, mineral-scale fouling is usually not a challenge, unless the source seawater pH has to be increased to 8.8 or more in order to enhance boron removal by the SWRO membranes. Calcium carbonate is the most commonly encountered mineral foulant in brackish water desalination plants. Calcium sulfate and magnesium hydroxide are the most frequent causes of SWRO membrane scaling. Scale formation can be prevented by the addition of an antiscalant or dispersant to the source water.
It is important to note that although the source seawater's temperature usually has a limited influence on scale formation, when this temperature exceeds 35°C, calcium carbonate scale will form at an accelerated rate.
9.2 Parameters and Measurement Methods
Commonly used parameters which can be used to predict a source water's potential to form mineral scale of calcium carbonate are the Langelier Saturation Index (LSI) and the Stiff-Davis Saturation Index (SDSI). These indices are functions of the source seawater's pH, calcium concentration, alkalinity, temperature, and TDS concentration or ionic strength.
The Langelier Saturation Index can be calculated using the following equation:
LSI = pH – pHs (2.2)
where pH = the actual pH of the saline source water
pHs = (9.30 + A + B) - (C + D) (2.3)
A = [log(TDS - 1]/10, TDS in mg/L
B = -13.2 × log(temperature + 273) + 34.55, temperature in °C
C = log[Ca2+] - 0.4, [Ca2+] in mg/L as CaCO3
D = log(alkalinity), alkalinity in mg/L as CaCO3
The LSI value is indicative of the source water's ability to form calcium carbonate scale only, and is not reflective of the formation of other scalants. If LSI is higher than 0.2, the source water is likely to cause slight scaling; if it is above 1, the source water will cause severe scaling on membranes. If LSI is negative, the water has a tendency to dissolve scaling.
The LSI value predicts the scaling potential of the source water only for a TDS lower than 4000 mg/L. For saline source waters of higher TDS, the Stiff-Davis Saturation Index is applied. This index can be calculated as follows:
SDSI = pH - pCa - palk - K (2.4) where pCa = -log[Ca2+], [Ca2+] in mg/L as CaCO3
palk = -log(alkalinity), alkalinity in mg/L as CaCO3
K = a constant which is a function of total ionic strength and temperature
Values of K, as well as nomographs and examples for calculating LSI and SDSI, are presented elsewhere (American Water Works Association, 2007).
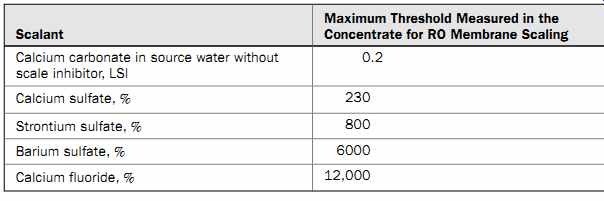
Table 6 Threshold Levels of Common Scalants
9.3 Threshold Levels of Mineral Foulants
Table 6 presents the concentration threshold levels of common mineral scalants above which the compounds will begin to accumulate on the membrane surface and form mineral scales (Chemical Pretreatment for RO and NF, 2008). The mineral scaling potential of the saline source water can be determined using proprietary software available from the manufacturers of antiscalants. The topic of scale formation and prevention is discussed in greater detail in section 9.
10. Natural Organic Foulants
10.1 Description
Depending on their origin, all saline waters can contain naturally occurring or man-made organic compounds and aquatic microorganisms. Since all microorganisms and most organic molecules are relatively large in size, they are well rejected by RO membranes. However, some organic compounds and aquatic species may accumulate on the membrane surface and form a cake layer that can significantly hinder the membrane's main function-rejection of dissolved solids. Depending on the main source of the fouling cake layer, these RO membrane foulants are typically divided into two separate groups: natural organic foulants and microbiological foulants (or biofoulants). The phenomenon of accumulation of aquatic organisms and their metabolic products on the membrane surface is known as biological fouling or biofouling.
Natural organic matter (NOM) is typically contained in surface saline waters (brackish or open ocean seawater) and includes compounds which are produced by naturally decaying algae and other aquatic vegetation and fauna: proteins, carbohydrates, oils, pigments (i.e., tannins), and humic and fulvic substances (acids). High NOM content in the source water used for production of drinking water is undesirable because it causes discoloration of the water, forms carcinogenic disinfection byproducts when disinfected with chlorine, and results in complexation with heavy metals, which in turn causes accelerated membrane fouling.
Under normal non-algal-bloom conditions, typical seawater and brackish waters collected using open intakes do not contain quantities of NOM great enough to present a significant challenge to desalination plant operations. High NOM content is usually observed during algal blooms and when the desalination plant intake is located near a confluence with a river or other freshwater source or near wastewater treatment plant discharge. The easily biodegradable organic matter released by algae during their growth and respiration is referred to as extracellular organic matter. When algae die off during the end phase of an algal bloom or their cells are broken by treatment or pumping processes, they also release intracellular organic matter in the source water. The combination of extracellular and intracellular organic matter from algae is referred to as algogenic organic matter (AOM). This NOM is easily biodegradable and provides a food source for biogrowth of bacteria on the RO membrane surface.
Humic acids are polymeric (polyhydroxyl aromatic) substances which have the ability to form chelates with metal ions in the water, such as iron. This feature of humic acids is very important for seawater or surface brackish water pretreatment systems using iron coagulants, because the humic acids can form a gellike layer of chelates on the surface of the membranes, which would cause fouling. Typically, such a fouling layer can be dissolved at a pH of 9 or more, at which condition both the membranes and the humic substances carry a negative charge. This feature is used for membrane cleaning.
Humic substances are hydrophobic, and therefore hydrophilic membranes are less prone to fouling by humic acids.
Most NOM in seawater consists of compounds of relatively large molecular weights (500 to 3000 daltons). A typical SWRO desalination membrane would reject over 90 percent of compounds that have molecular weight higher than 200 Da.
Humic and fulvic substances mainly differ in their ability to dissolve in strong acids. While humic substances are easily precipitated upon acidification of the source water, fulvic substances remain in solution at low pH.
Humic acids in their natural state are not a food source for most aquatic organ isms. However, when oxidized with chlorine or other oxidants, they can become easily biodegradable and serve as a food source for aquatic bacteria growing on the RO membrane surface. Therefore, continuous chlorination of source water containing large amounts of humic acids often causes more membrane biofouling problems than it solves.
Negatively charged NOM, which dominates in surface brackish water and seawater collected by open intakes, has a tendency to adhere to the surface of thin-film composite RO membranes. Once adsorption occurs, the NOM begins to form a cake or gel on the membrane surface and to affect membrane performance. It should be noted that NOM, depending on its properties and origin, may also adhere to the surface of UF and MF pretreatment membranes and cause significant productivity loss by plugging membrane pores, adsorbing to the internal matrix of the membranes, and forming a cake of organic matter on the membrane surface. Usually, saline water from surface water sources contains NOM that causes moderate fouling of UF and MF membranes which can be removed by routine chemically enhanced backwash and periodic cleaning of the pretreatment membranes.
This NOM can be removed from UF and MF membranes with very little (typically less than 2 mg/L) or no coagulant addition to the saline source water. However, if the source water is influenced by surface runoff or a large amount of alluvial organics, or if an alluvial brackish aquifer is used for water supply, the NOM's properties and ability to cause significant membrane productivity loss may increase dramatically. Under these circumstances, the efficient removal of NOM may require very high dosages of coagulant (usually over 20 mg/L).
A NOM fractionation study on surface waters completed by the U.S. Bureau of Reclamation in 2002 indicates that this type of fouling depends on the type of membrane material and characteristics, the polarity and molecular weight of the NOM, and the chemistry of the source water.
The largest natural organic foulants are the polysaccharides, organic colloids, and proteins, followed in size by humic substances, organic acids, and low-molecular weight organics of neutral charge. These compounds have different potentials to cause membrane fouling. Polysaccharides excreted by living bacteria on the membrane surface have the highest potential to cause RO membrane biofouling, and therefore they are classified as a separate group of foulants-microbial foulants.
10.2 Parameters and Measurement Methods
The most frequently used parameters for quantification of organic fouling are total organic carbon, UV254 , and biofilm formation rate.
Total Organic Carbon
Total organic carbon (TOC) is one of the most widely used measures for the organic content of saline source water. TOC concentration measures the content of both NOM and easily biodegradable organics, such as polysaccharides, released during algal blooms. This water quality parameter is widely used because it is relatively easy to measure and it is indicative of the tendency of the source water to cause organic fouling and biofouling of an RO membrane. TOC is measured by converting organic car bon to carbon dioxide in a high-temperature furnace in the presence of a catalyst.
Typically, open ocean seawater which is not influenced by surface freshwater influx (a nearby river confluence), human activities (i.e., wastewater or storm water discharges or ship traffic), or algal bloom events (i.e., red tide) has a very low TOC content (= 0.2 mg/L). When an algal bloom occurs, however, the TOC concentration of ocean water can increase by an order of magnitude or more (2 to 12 mg/L). A similar magnitude of TOC increase could be triggered by a storm water or river discharge during a high-intensity rain event, such as those that occur during rainy seasons in tropical and equatorial parts of the world. Usually, an increase of TOC content in the source water above a certain threshold (2.0 to 2.5 mg/L) triggers accelerated biofouling of RO membranes.
Observations at the Carlsbad seawater desalination demonstration plant in California, USA (which is supplied by seawater collected using near-shore open ocean intake), indicate that when TOC concentration in the source water at that location exceeds 2.0 mg/L during algal bloom events, within a one- to two-week period the SWRO system experiences measurable biofouling and an associated increase in operating pressure.
Similar TOC-level observations at the Tampa seawater desalination plant in Florida, USA (where the typical background TOC level of the seawater is less than 4 mg/L) indicate that accelerated biofouling occurs when the TOC concentration exceeds 6 to 8 mg/L. Usually, accelerated biofouling at the Tampa facility is triggered by one of two key events--rain events, which increase the content of alluvial organics in the source seawater, or algal blooms, which cause elevated organic levels due to massive dying off of algae. The increase in alluvial organics during rain events is caused by the elevated flow and alluvial content of the Alafia River, which discharges into Tampa Bay several kilometers upstream of the desalination plant's intake. During high-intensity rains in the summer months, the TOC level in the river water discharging in the bay may exceed 20 mg/L.
Analysis of various sources of seawater indicates that TOC in seawater may contain various fractions of organics, depending on the origin of the water and the type of seawater intake (Table 7). These fractions may also change depending on the season. Review of Table 7 leads to the conclusion that low-molecular weight organic compounds are typically the greatest fraction of the TOC in seawater (at least 40 percent). Most of these compounds, however, have limited fouling potential; therefore, the higher the percentage of low-molecular-weight compounds in the source water, the lower the water's fouling potential is. A combination in a source water of a high percentage of compounds from the "other low-molecular-weight" category, low TOC, and low polysaccharide content is a clear indication of low fouling potential.
Based on this rule of thumb, the well seawater at the Gibraltar SWRO facility would have the lowest fouling potential among all sources listed in Table 7.
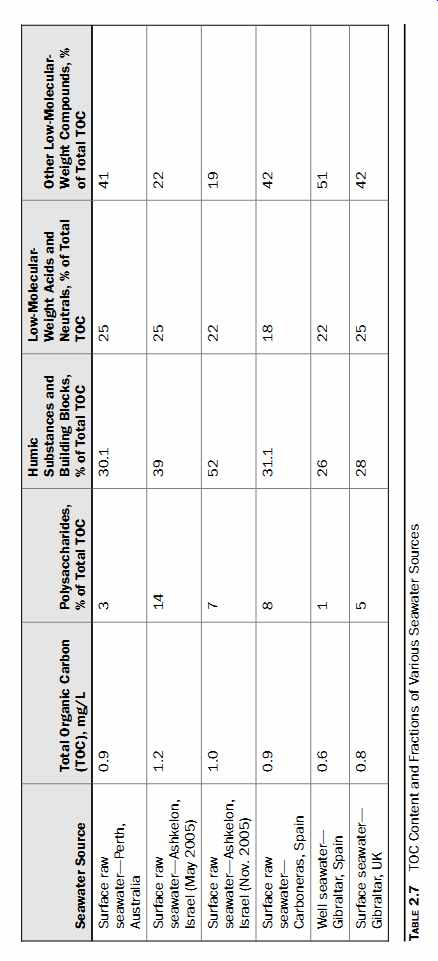
Table 7 TOC Content and Fractions of Various Seawater Sources
Comparison of the data from the Ashkelon seawater desalination plant in Israel indicates that the most easily biodegradable organics (polysaccharides) change season ally, increasing during the summer season along with the content of algal biomass in the Mediterranean Sea. These data also show that the TOC concentration of seawater may not always correlate with the content of polysaccharides in the water. In this case, humic substances are the main contributor of fouling of the SWRO membranes at the Ashkelon plant.
UV254 Absorbance
The ultraviolet (UV) absorbance of a seawater sample at 254 nm is an indirect measure of NOM. The UV254 absorbance of a saline water sample is determined by filtering the sample through a 0.45-µm filter and measuring the filtrate's absorbance of UV with a spectrophotometer. This measurement is based on the fact that specific molecular structures (chromophores) within the NOM molecules absorb UV light.
Because the NOM composition may vary from one water source to another, UV254 absorbance is not always easy to use for comparing the fouling potentials of different water sources. In addition, this parameter may not be reflective of the content of microbial foulants if the NOM contained in the source water is not easily biodegradable.
Specific UV absorbance (SUVA) is defined as the UV absorbance divided by the concentration of dissolved organic carbon in the source water. Edzwald and Haarhoff (2013) indicate that SUVA can be used as an indirect indicator of the occurrence of algal blooms in the source water. If the SUVA is higher than 4, then NOM in the source water consists predominantly of aquatic humic matter and it is not exhibiting algal bloom conditions. If the SUVA is between 2 and 4, the source water's NOM is a mix of assimilable organic matter (AOM) and aquatic humic matter, and the source water body from which the water originates is in the early stages of an algal bloom. When the SUVA is less than 2, the NOM in the source water consists predominantly of AOM and the source water body is experiencing an algal bloom.
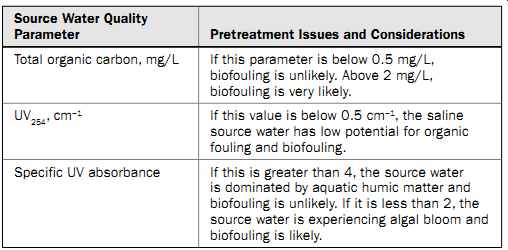
Table 8 Water Quality Parameters for Characterization of Organic Foulants
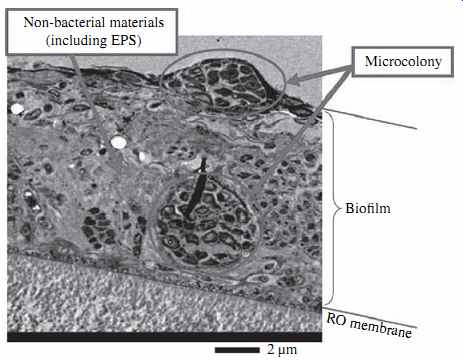
FIG. 3 Biofilm on the surface of an RO membrane. (Source: Nitto Denko.)
10.3 Threshold Levels of Organic Foulants
Table 8 presents threshold levels of key water quality parameters used for assessing the organic fouling potential of saline source water.
11. Microbial Foulants
11.1 Description
Microbial foulants are aquatic microorganisms and organic compounds excreted by them (i.e., extracellular polymeric substances, proteins, and lipids) which are deposited on the surface of RO membranes. The biofilm formed on the membrane surface (FIG. 3) contributes additional resistance (pressure head losses) to the osmotic pres sure that must be overcome in order to maintain steady production of freshwater by the membrane elements.
Recent research indicates that a biofilm accumulated on the surface of RO membranes can cause performance decline by increasing the hydraulic resistance of the membranes and by a "cake enhanced osmotic pressure" effect. Therefore, if a microbial cake layer is formed on the surface of the membranes, membrane productivity (flux) declines and membrane salt passage increases over time. In order to compensate for the loss of productivity due to biofouling, the feed pressure of the RO membrane system would need to be increased, which in turn would result in elevated energy use to produce the same volume of freshwater. Feed pressure increase beyond a certain level may cause irreversible damage to the membrane structure and ultimately may result in the need to replace all RO membrane elements.
Although bacteria constitute the majority of the membrane biofilm, other microorganisms such as fungi, algae, and protozoa can also attach to the membrane surface and contribute to biofilm formation. Usually the most predominant bacteria causing bio fouling are Pseudomonas, Bacillus, Arthrobacter, Corynebacterium, Flavobacterium, and Aeromonas. Other microorganisms such as fungi (e.g., Penicillium, Trichoderma, Mucor, Fusarium, and Aspergillus) are typically present in the membrane biofilm in significantly lower levels than bacteria.
Biofouling is usually a significant operational challenge for saline waters of naturally elevated organic content and temperature (such as the seawater in the Middle East). Biofouling is also a challenge during intense algal blooms (i.e., red tides) or periods when surface runoff from rain precipitation or nearby river water of high organic content enters the plant's open intake. The biofouling potential of a given source water depends on many factors, including: (1) the concentration and speciation of microorganisms contained in the source water, (2) the content of easily biodegradable compounds in the water, (3) the concentration of nutrients and the balance (ratio) between organic compounds and the biologically available nitrogen and phosphorus in the source water, and (4) the source water temperature.
Bacteria contained in brackish water and seawater typically exist in two states- metabolically active and inactive. The active state of bacterial cells is characterized by fast growth and the formation of extracellular material and bacterial colonies that can accumulate on an RO membrane surface. The inactive state of existence of aquatic bacteria is characterized by low metabolic and growth rates; the cells appear in the form of single cells or small cell clusters that behave as microparticles and have a protective cellular cover which allows them to survive unfavorable environmental conditions such as a low content of food and oxygen or a high content of harmful substances such as chlorine and other biocides. At any given time, some of the aquatic bacteria naturally occurring in surface water bodies are in an active state and others are in an inactive state.
The predominant state of aquatic bacteria (active or inactive) depends on how favor able the ambient environment is for bacterial survival and growth. Most aquatic bacteria will transfer from an inactive to an active state under favorable environmental conditions, such as algal bloom events, when high concentrations of easily biodegradable organics released from the decaying algal biomass (which serve as food to these bacteria) are readily available in the source water. Since bacteria can attach on a membrane surface and grow colonies there at a very high rate, red tide or other intense algal bloom events are usually the most frequent cause of RO membrane biofouling, especially in seawater desalination plants.
The membrane biofouling process (i.e., the formation of a microbial cake layer on the surface of an RO membrane) usually follows several key steps: (1) formation of a primary organic conditioning film, (2) attachment of colonizing bacteria, (3) formation of a biopolymer matrix, (4) establishment of a mature secondary biofilm, and (5) biofilm equilibrium and die-off. The primary organic conditioning film is a microthin layer on the surface of the membrane that is rich in nutrients and easily biodegradable organics, and that creates suit able conditions for bacteria to convert from an inactive (particulatelike) state into an active state. In this state they are capable of producing extracellular polysaccharides, which are adhesive substances that allow bacteria to attach to the membrane surface and to each other.
During the first step of the biofilm formation process, active bacteria adsorb to only 10 to 15 percent of the membrane surface, but they multiply at an exponential rate, and within 5 to 15 days they colonize the entire membrane surface and form a biopolymer matrix layer that is several micrometers thick. The mucoid biopolymer matrix formed on the membrane surface entraps organic molecules, colloidal particles, suspended sol ids, and cells of other microorganisms (fungi, microalgae, etc.) over time to form a thicker cake with a higher resistance to permeate flow.
In order for biological fouling to occur, aquatic bacteria need to have suitable low-velocity conditions to attach to the RO membrane surface or to the surface of facilities upstream of the membrane system, such as cartridge filters; or they need low-velocity cavities or vessels along the feed water route to the RO membrane system, such as dead-end valves, fittings, or oversized or hydraulically flawed cartridge filter housings.
The formation of a permanent cake layer occurs when membrane flux exceeds a certain level (critical flux) at which aquatic microorganisms can attach to the RO membrane surface. When critical flux through the membrane is reached, the velocity of the feed water/concentrate flow along the surface of the membrane (cross-flow velocity) drops low enough to allow colonizing bacteria to attach to the membrane surface.
The critical flux for aquatic bacteria is dependent upon the cross-flow velocity and increases with the increase of this velocity. The most widely used operational approach to increase cross-flow velocity is to reduce RO system recovery. Operating at lower recovery leaves more flow on the concentrate/feed side of the membranes, which in turn creates a higher scouring velocity on the membrane surface that deters microorganisms from attaching to the surface.
The critical flux is also a function of the concentration of active bacteria in the source water; and it decreases as the concentration of bacteria rises. The concentration of active aquatic bacteria in turn mainly depends on the type of bacteria, the availability of easily biodegradable organic matter in the source water, and the water temperature. For a given SWRO system, decreasing recovery from 50 to 35 percent would result in approximately two times lower fouling potential for system operation in a typical flux range of 13.5 to 18.0 L/(m2·h) [8.0 to 10.5 gal/(ft2·d) (gfd)].
Although operation at low recovery may be attractive from the point of view of minimizing membrane biofouling, designing RO plants for low recovery is usually not cost effective because of the associated increased size of the desalination plant intake, pretreatment, and RO systems, and the 30 to 40 percent higher capital costs. Therefore, other approaches for biofouling reduction-such as control of the organic content in the source water and inactivation of aquatic bacteria by disinfection or UV irradiation- have found wider practical application.
The source of biofouling may not only be a natural event (such as an algal bloom) that triggers an increase in the content of easily biodegradable organics in the source water; it may also be the type and operation of the pretreatment processes and systems used upstream of the RO facility. One reason for accelerated biofouling could be continuous chlorination of the source water, which often is applied to inactivate aquatic microorganisms and reduce biofouling. Since chlorine is a strong oxidant, it can destroy the cells of active aquatic bacteria and algae which naturally occur in the source water at any given time.
The destroyed algal and bacterial cells release easily biodegradable organic com pounds (such as polysaccharides) in the ambient water, which become food for the remaining aquatic bacteria that have survived chlorination by being in an inactive state. If the concentration of these organics reaches a certain threshold, it could trigger the conversion of these surviving bacteria from an inactive to an active state, followed by their attachment and excessive growth on the RO membrane surface, which in turn would manifest as membrane biofouling. Therefore, continuous chlorination often creates more membrane biofouling problems than it solves. On the other hand, intermittent chlorination has been found to provide effective control of 37 microbial growth without generating a steady influx of easily biodegradable organics that can trigger a large-scale transfer of aquatic bacteria from an inactive to an active state of existence.
Another pretreatment technology that could potentially cause biofouling, especially during periods of severe algal bloom events, is the use of pressure-driven granular media filters, UF, or MF membrane filters for pretreatment. Although pressure filters provide effective removal of particulate and colloidal foulants, the high filtration driving pressure applied by these systems could break some of the algal cells in the source water and cause the release of easily biodegradable organics, which in turn could result in accelerated RO membrane biofouling. Examples of seawater algal species susceptible to cell breakage as a result of relatively low pressure (0.3 to 0.6 bar; 4 to 8 lb/in2 ) are shown in FIG. 4.
From the point of view of minimizing biofouling associated with algal cell break age, the most suitable pretreatment technologies are those that provide a gentle removal of the algal cells in the source water, such as downflow gravity granular media filtration and dissolved air flotation.
Another potential source of biofouling is the use of impure source water conditioning chemicals, such as antiscalants, polymers, or acids. Therefore, it is important to analyze these chemicals for easily biodegradable organic content.
=======
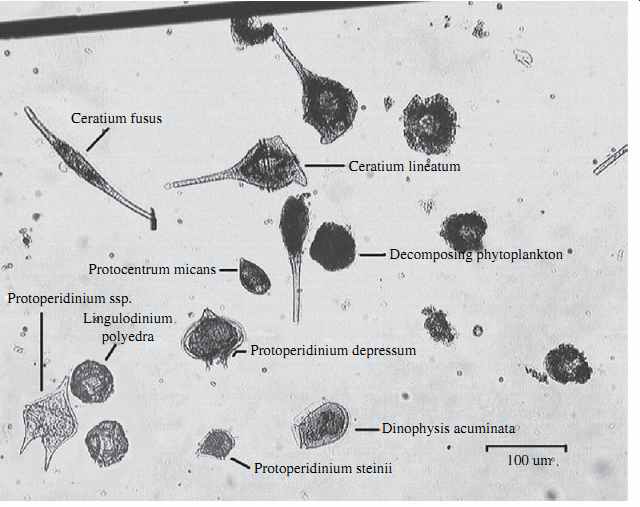
FIG. 4 Seawater algal species susceptible to cell breakage.
Ceratium fusus Protocentrum micans Protoperidinium ssp.
Protoperidinium depressum Decomposing phytoplankton Ceratium lineatum Dinophysis acuminata 100 um Protoperidinium steinii Lingulodinium polyedra
======
11.2 Parameters and Measurement Methods
Biofilm Formation Rate
The biofilm formation rate (BFR) is an online monitoring tool that allows operators to measure the accumulation of biomass on the surface of a glass ring as a function of time; it is measured in picograms of adenosine triphosphate (ATP) per square centimeter (pg-ATP/cm2 ; Vrouwenvelder and Van der Krooij, 2008). If BFR exceeds 120 pg-ATP/ cm2 and AOC is higher than 80 µg/L, severe biofouling is expected to occur. For a source water with BFR lower than 1 pg-ATP/cm2 , membrane biofouling is not expected to be a challenge. Veza and colleagues (2008) have found a correlation between the concentration of ATP biomass and the heterotrophic plate count of seawater.
11.3 Threshold Levels of Microbial Foulants
Table 8 includes a summary of measures commonly used to assess biofouling caused by microbial foulants. The most commonly measured source water quality parameters for characterization of biofouling caused by bacterial foulants are TOC and UV254. While other parameters such as BFR have been developed, they are not widely used because of the complexity of the source water quality analysis and the monitoring equipment needed to complete the tests.
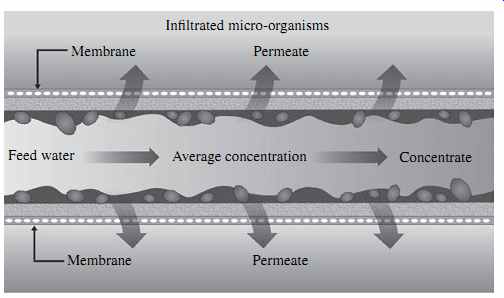
FIG. 5 Biofilm accumulation in the spacer of an RO membrane.
12. Combined Impacts of Various Types of Foulants
Membrane fouling is a complex process which often results from the additive impacts of several types of foulants. Particulate fouling usually causes a relatively quick and definitive deterioration of membrane permeate flux and RO system productivity, without significant impact on salt rejection. For comparison, colloidal fouling typically causes a marked deterioration in the RO system's salt rejection over time. In addition to deterioration of salt rejection, colloidal fouling also results in permeate flux decline over time, which is caused not only by the accumulation of a flow-resistant cake layer of colloidal particles on the surface of the membrane but also by back fusion of salt ions within the colloidal cake, which results in elevated salt concentration and osmotic pressure at the membrane surface; this in turn decreases the net driving pressure. In contrast, fouling caused by NOM is accompanied by almost constant salt rejection over time.
Because of its complex nature, NOM often creates diverse interactions with other foulants and with the surface of the membrane elements. As indicated previously, hydrophobic humic substances are typically a major foulant in source seawater, especially when the seawater is under the influence of river discharge. The high content of calcium in seawater reduces the solubility of humic acids and increases their aggregation, which in turn accelerates the accumulation of these NOM compounds on the surface of the RO membranes.
The calcium complexation of NOM in this case often forms a gel on the surface of the membranes, which is very difficult to remove. Therefore, source seawater originating from an area of river confluence into the ocean-especially if the river water were high in NOM-would be very difficult to treat and use for membrane desalination.
Biofouling of the feed channels and spacers of spiral-wound membranes ( FIG. 5) typically results in a significant increase in the membrane differential pressure (i.e., the pressure difference between the feed and concentrate sides of the membranes) over a very short period of time (one to several weeks).
As biofilm-forming bacteria colonize the RO membrane surface, they often block sections of the feed channels and spacers between the membrane leaves over time, so that the flow pattern within the membrane elements changes and the feed flow is completely blocked in some portions of the feed channels and increased in others. Flow channeling caused by random blockages of the feed channels and spacers results in a sharp increase in salt concentration in the affected areas, which in turn triggers precipitation of sparingly soluble salts such as calcium carbonate and sulfate in the feed channels and further exacerbates and accelerates the membrane fouling problem.

FIG. 6 Autopsy of a fouled RO membrane.
13. Membrane Fouling Diagnostics
13.1 Laboratory Autopsy of Biofouled Membranes
A laboratory autopsy is a method of characterizing the fouling nature of source water constituents that is applied when the fouling process has already taken place; it is typically used when the source water contains a number of different types of foulants and it is difficult or practically impossible to determine the source of fouling by analyses of source water quality and RO system performance only. Standard laboratory autopsy investigation involves the dissection of a fouled membrane element and physical observation of the condition of the membrane leaves and spacer ( FIG. 6).
A sample is collected from the front end of the membrane for microbial and chemical analysis. Results are expressed in colony-forming units per square centimeter (cfu/cm2 ) and by foulant composition, respectively.
The moisture content and the chemical composition of the dried deposit of the collected biofilm sample are expressed as a percentage of the foulant. Often, samples are inspected using scanning electron microscopy and surface analysis such as x-ray photoelectron spectroscopy, especially if the predominant fouling is of a complex multicause nature.
14. Water Quality Analysis for RO Desalination
Based on the discussion of the factors influencing RO system performance presented in the previous sections, it is recommended that, at a minimum, the source water for a given RO desalination project be analyzed for the parameters listed in Table 9.
Besides key minerals and parameters associated with various types of membrane fouling, Table 9 also contains water quality characteristics that may have an impact on the performance, integrity, and longevity of the RO membranes, such as pH, free chlorine, ammonia, and oxidation reduction potential; metals which if present above certain thresholds could induce membrane oxidation and integrity loss, such as copper, and iron. The list presented in Table 9 is not all-inclusive. Additional source water quality parameters may need to be measured, depending on the project and site-specific regulatory requirements governing source water intake, product water quality, and concentrate discharge.
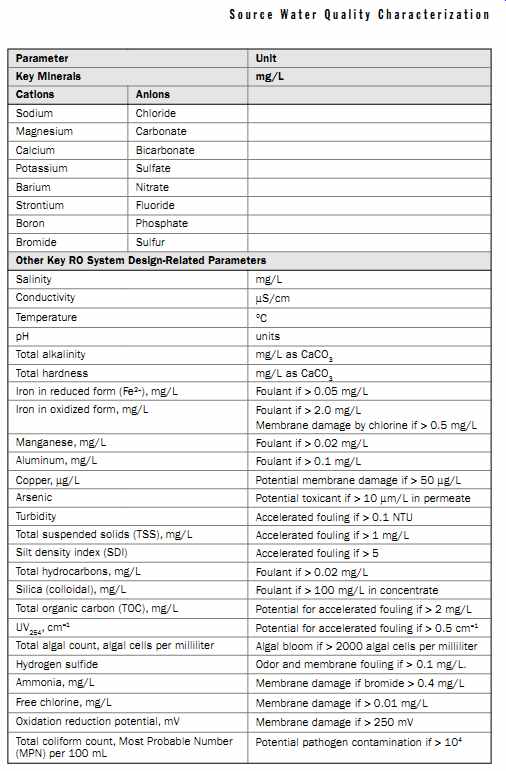
Table 9 Source Water Quality Analysis for Reverse Osmosis Desalination
15. References
American Water Works Association, Manual of Water Supply Practices, M46, Reverse Osmosis and Nanofiltration, 2d ed., American Water Works Association, Washington, D.C., 2007.
Boerlage, S. F. E., "Understanding SDI and Modified Fouling Indices (MFI0.45 and MFLUF )," Proceedings of IDA World Congress, Masspalomas, Gran Canaria, Spain, October 21-26, 2007. Ref: IDAWC/MP07-143.
Chemical Pretreatment for RO and NF, Technical Application Bulletin No. 111, Revision C, Hydranautics, Oceanside, California, 2008.
Edzwald, J. K., and J. Haarhoff, "Seawater Pretreatment for Reverse Osmosis: Chemistry, Contaminants and Coagulation," Water Research, 45 (17): 5428-5440, 2011.
Herzberg, M., and M. Elimelech, "Biofouling of Reverse Osmosis Membranes: Role of Biofilm-Enhanced Osmotic Pressure," Journal of Membrane Science, 295: 11-20, 2007.
Khirani, S., B. R. Aim, and M. H. Manero, "Improving the Measurement of the Modified Fouling Index Using Nanofiltration Membranes (NF-MFI)," Desalination, 191: 1-7, 2006.
Konishi, T., K. Maruyama, M. Namba, M. Kobuke, M. Nozoe, E. Liu, T. Akiyama, et al., "LD (Low-Differential-Pressure) Technology for Biofouling Control-Design Concept Supported by Microbiological Analysis," Proceedings of IDA World Congress, Perth, Western Australia, September 4-9, 2011. Ref: IDAWC/PER-081, 1-9.
Leparc, J., S. Rapenne, C. Courties, P. Lebaron, J. P. Croute, V. Jacquemet, and G. Tyrner, "Water Quality Performance Evaluation at Seawater Reverse Osmosis Plants Through The Use of Advanced Analytical Tools" Desalination, 203: 243-255, 2007.
Mosset, A., V. Bonnelyle, M. Petry, and M. A. Sanz, "The Sensitivity of SDI Analysis: From RO Feed Water to Raw Water," Desalination, 222: 17-23, 2008.
US Bureau of Reclamation, Membrane Fouling: Influence of Natural Organic Matter Properties and Membrane Surface Treatment on Nanofiltration Treatment, Desalination and Research Development Report No. 83, US Bureau of Reclamation Washington, D.C., 2002.
Veza, J. M., M. Ortiz, J. J. Sadhwani, J. E. Gonzalez, and F. J. Santana, "Measurement of Biofouling in Seawater: Some Practical Tests," Desalination, 220: 326-334, 2008.
Vrouwenvelder, J. S., and D. Van der Krooij, "Diagnosis, Prediction, and Prevention of Biofouling on NF and RO Membranes," Desalination, 139: 65-71, 2008.
Winters, H. S., A. Fane, and B. Nicolaisen, "Microbial Membrane Fouling; Microbial Cake Enhanced Osmotic Pressure (CEOP) or Thermodynamic Restriction?," Proceedings of IDA World Congress, Masspalomas, Gran Canaria, Spain, October 21-26, 2007.
Ref: IDAWC/MP07-267.