58. What is a fertilizer?
Any material that contains some nutrients needed for plant growth. It can be natural material, such as manure, or it can be a manufactured material, such as ammonium nitrate. See Table 3-1 below for a listing of natural and manufactured materials.
Natural Materials Bone meal Cocoa shells Grass clippings Farm manure Cotton hulls Guano |
Manufactured Materials Ammonium nitrate Ammonium sulfate Superphosphate Ureaform Potash Urea |
59. What is the difference between inorganic, natural organic, and synthetic organic fertilizers?
Inorganic fertilizers are materials such as ammonium nitrate and potassium sulfate that are in a soluble salt form and that readily dissolve in water for plant use. Natural organic fertilizers are materials such as manure or guano that have to undergo decomposition to release the nutrients in a form that the plants can use. (See Table 3-2). Synthetic organic fertilizers are manufactured products that have the nutrients bonded into a carbon hydrogen complex. This usually results in a slower, more con trolled release of the nutrients over a period of time. In most cases this type of fertilizer is best for lawn use.
TABLE 3-2: Plant Food Value of Various Manures
|
% N – P - K |
Rate Nitrogen is Available |
Horse Cow Sheep Chicken |
3 – 2 - 2½ 4 - 1½ - 3 3 - 1½ - 3 6 – 4 - 2 |
very slow slow slow fast |
EXAMPLE: One hundred pounds of horse manure would contain pounds of nitrogen (N), 2 pounds of phosphorus (P), and 2½ pounds of potassium (K).
60. How much nitrogen is there in the natural organic fertilizers?
The nitrogen content will vary, but it is usually very low (1.0 percent—6.o percent). A large volume of material is therefore needed to supply the grass with sufficient nitrogen.
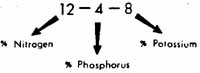
61. What do the numbers 12-4-8 represent on a fertilizer bag?
These numbers refer to the percentage of nitrogen, phosphor us, and potassium in the bag. A 50 pound bag of 12—4—8 fertilizer would contain 6 pounds of nitrogen, 2 pounds of phosphorus, and 4 pounds of potassium. The other 38 pounds are carrier material. (See fig. 3-1.)
Figure 3-1. Relative percentages of the three major nutrients in fertilizer.
62. How can I tell if the fertilizer I am buying is actually for lawn use?
Usually if the fertilizer bag has the words “turf or lawn fertilizer” on it or gives the rates that the fertilizer should be put on a lawn in terms of pounds per 1,000 square feet, then chances are good it was made for use on lawns. Another check is to look at the grade (12—4—8, 18—5—9, 16—4—8) on the fertilizer bag. If the nitrogen is approximately twice as high as the potassium then it is probably a turf fertilizer. Farm-brand fertilizers that have all three numbers of the grade the same (12—12—12), or whose nitrogen source is all water-soluble, are not the best for use on home lawns. However, if this is all that is available to you, it is better than no fertilizer at all.
63. What do people mean when they speak of a fertilizer ratio and grade?
The term grade refers to the percentage of nitrogen, phosphorus, and potassium present in the bag of fertilizer. The term ratio means the proportion of these three major nutrients to one another in a bag of fertilizer. In general, the best fertilizers for lawn use have a ratio where the nitrogen is 2 to 3 times higher than the potassium. There should be less phosphorus present in the bag than potassium. See Table 3-3.
TABLE 3-3: Fertilizer--Grades and Ratios
12—12—12 16—8—8 16—4—12 20—5—10 24—4—8 |
1:1:1 2:1:1 4:1:3 4:1:2 6:1:2 |
64. What do the terms complete fertilizer and balanced fertilizer mean when found on a fertilizer bag?
Complete fertilizers are those containing nitrogen, phosphorus, and potassium, but the amounts can vary considerably. Some examples of the different grades of complete fertilizer you can buy are 16—8—8, 12—12—12, 16—4—8, 30—3—10. 18—5—9, and 16—6—4. A balanced fertilizer is one having the three major nutrients—nitrogen, phosphorus, and potassium—mixed in a ratio most beneficial for the grass. Balanced turf fertilizers would have grades similar to the following examples: 12—4—8, 18—5—9, and 30—3—10.
65. What are the important plant nutrients a lawn fertilizer should contain?
Nitrogen, phosphorus, and potassium are the three major nutrients to look for. If other nutrients are listed, consider them a bonus.
66. Why do the grass plants need nitrogen, phosphorus, and potassium?
Nitrogen causes the grass to have a deep-green color. It also stimulates good growth of the leaf and blades, producing a luxurious lawn. Phosphorus is primarily used in developing a good root system. Potassium enables the plant to take up nutrients and gives the plant resistance to diseases.
67. Is it always necessary to use a turf fertilizer containing nitrogen, phosphorus, and potassium?
Most lawns are under-fertilized. Using a complete fertilizer would certainly be beneficial. If you are sure there is an adequate supply of phosphorus and potassium in your soil, you could use a fertilizer containing only nitrogen. It would be a good idea, how ever, to apply a complete fertilizer to your lawn at least once a year.
68. Does the term minor element on a fertilizer bag mean the nutrients are not as important for plant growth as are the major nutrients (nitrogen, phosphorus, and potassium)?
The minor elements (nutrients) are as important for plant growth as are the major nutrients. The terms minor and major refer to the amount needed by the plants to complete a life cycle. Table 3-4 shows a list of major and minor nutrients.
Major Nutrients Carbon Calcium Hydrogen Magnesium Nitrogen Oxygen Phosphorus Potassium Sulfur |
Minor Nutrients Boron Chlorine Copper Iron Manganese Molybdenum Zinc |
69. Fertilizer is an added expense. Is it necessary?
Because fertilizing is one of the keys to having a nice lawn, it should be done. It will cause the grass to be a deep-green color, help it withstand heavy use, and keep the weeds out. With fertilization, a lawn will have a better chance of maintaining itself against all the other competition (weeds, disease, and insects).
70. What month of the year should a fertilizer be applied?
This will be determined by the weather, type of grass, and amount of water available. Grass should be fertilized at the beginning and end of the growing season. An application in the middle of the season is good if the weather is not too hot and there is water for irrigation. (See chapter ii for additional information).
71. How does fertilizing in the early spring benefit my lawn?
The most important thing it does is to insure the availability of an adequate supply of nitrogen to the grass plant. This will help get your grass off to a quick start and allow it to green-up quickly in the spring. This will not only result in an attractive lawn but will also give the lawn a good head start on any weeds that are trying to get established.
72. How much fertilizer should be applied to a lawn in a single year?
This depends on the grade of fertilizer being used. Generally the rates are given as pounds of actual nitrogen to be applied per 1000 square feet per year. Bermuda-grass likes 8 to 12 pounds actual nitrogen per 1000 square feet per year, whereas bluegrasses prefer 4 to 8 pounds actual nitrogen per 1000 square feet per year. The fertilizer applications should be divided over the entire year. See Table 3-5 for nitrogen requirements for lawn grasses.
TABLE 3-5: Seasonal Nitrogen Requirements for Lawn Grasses
Grasses Bahiagrass Bentgrass Bermudagrass Carpetgrass Centipedegrass Chewing Fescue Kentucky Bluegrass Red Fescue Ryegrass St. Augustine Tall Fescue Zoysia |
Pounds of nitrogen per 1000 sq. ft. 4—5 6—S 8—12 3—4 3—4 4—6 4—8 4—6 4—6 4—6 3—4 5—6 |
73. How should a fertilizer be applied?
It is essential to have uniform coverage, not only to insure good plant growth but also to avoid those embarrassing signs of mis application of a fertilizer. The worst possible way is to fill a bucket with fertilizer and walk around the lawn tossing out handfuls at random. Incorrect fertilizing will show up quickly. Whenever you see dark-green strips in a lawn, with light-yellow strips between, you can be certain that uniform coverage of the lawn was not taken into consideration. Using a cyclone spreader is the best way to insure proper coverage of a lawn. If the drop-type spreader is used, more care must be used to avoid misapplication.
74. How can a fertilizer burn grass?
The term fertilizer burn refers to the situation where a granule of fertilizer (soluble salts) rests directly against the plant. The high salt concentration draws moisture from the plant cells in contact with the granule, and the cells die because of their dehydrated condition.
How to Avoid Fertilizer Burn to the Grass Plant
1. Never apply more than two pounds of nitrogen per 1000 square feet at any one time.
2. Be sure to spread fertilizer material evenly over the lawn.
3. Do not overlap the fertilizer material when applying it or spill fertilizer on the grass.
4. Apply the fertilizer when the grass is dry.
5. Water as soon as possible after fertilizing. Try to time your fertilizer application just before a gentle rain.
75. Is it necessary to water after fertilizing the lawn?
Watering the fertilizer into the soil is a good way to insure there is no damage done to the grass because of fertilizer burn. If possible, fertilize ahead of a rainstorm and let nature do your work.
76. Will fertilizing my lawn do any harm to the nearby trees and shrubs?
Usually it won’t harm them unless you get fertilizer on the leaves when they are wet. A well-fertilized lawn will usually sup ply enough nutrients to keep the trees and shrubs growing ac tively. You also need to be careful to keep the fertilizer away from your trees and shrubs if it contains a broadleaf weed killer. Re member, trees and shrubs are also broadleaf plants and are easily killed by the broadleaf herbicides.
77. If organic material (peat, sawdust, etc.) is added to my lawn, is it necessary to fertilize?
Yes, because the nitrogen in the soil will no longer be readily available for plant use. The bacteria in the soil will tie up the nitrogen for use in decomposing the excess organic matter. In this case it is often necessary to add extra nitrogen to a lawn so that the grass and the bacteria will have nitrogen available for their use.
78. I have put several applications of nitrogen fertilizer on my lawn, but it is still yellow. What is wrong?
If the grass has had ample nitrogen applied to it and remains a yellowish color, then chances are the nutrient you need to add is iron. Buy some iron sulfate from a local store and sprinkle some on a small area in your lawn. If the grass greens-up in 5 to 10 days, then you know iron was the missing nutrient.
79. What do the letters UF mean in a fertilizer advertisement?
This is an abbreviation for urea-formaldehyde, which is a synthetic organic nitrogen commonly used in lawn fertilizers.
80. After a lawn is fertilized, how much time must elapse before children and pets are allowed on the lawn?
If you are worried about the fertilizer doing any harm to the children and their pets, you shouldn’t be. Unless there is an insecticide mixed in with the fertilizer, there is very little chance of their being harmed. So far as the children or pets doing any dam age to a recently fertilized lawn, there is little to worry about so long as the grass was dry when the fertilizer was applied.
81. Does the type of soil in my yard have anything to do with how often the lawn needs to be fertilized?
(missing content)
89. I have a soil that is very acid (pH = 5.5, according to a soil test). Will this have any effect on how much fertilizer I should put on my lawn?
The pH of the soil determines the availability of the nutrients. Therefore any time the soil has a sour (acid) condition such as yours, the nutrients needed by the grass will be affected. The ideal soil pH is 6.0 to 7.6 for grasses; in this pH range the plants are able to utilize the nutrients from the soil. At a lower pH, phosphorus, potassium, and nitrogen aren’t as available to the plant, and if you don’t plan on liming, then you’ll have to apply a little extra fertilizer to overcome the effects of the acid soil.
90. My soil is slightly alkaline (pH = 7.9). How can I reduce the pH to about 7.0?
It is more difficult to lower the pH of a soil than to raise it. Any of the fertilizers having nitrogen in the form of ammonium, or other forms that break down to ammonium, can be used to lower the pH of your lawn. This way you not only supply the grass with the plant food it needs, but you also use the plant food in a form that will reduce the alkaline condition of your soil. Large amounts of elemental sulfur can be put on your lawn if you are in a hurry to change the pH of your soil. The following list of nutrients, when applied to your lawn, will help increase the acidity of the soil: ammonium sulfate, urea, ammonium phosphates, and urea- forms.
91. How can you determine how large an area a bag of fertilizer will cover?
To answer this question, let’s assume you have bought a 40-pound bag of fertilizer with the following analysis (grade), 30—3—10, and that you want to apply 2 pounds of nitrogen per 1,000 square feet. First, determine how much nitrogen there is in the bag by multiplying the percent of nitrogen by the weight of the bag (30 percent X 40 lbs. = 12 pounds of actual nitrogen in the bag of fertilizer). Since there are 12 pounds of nitrogen in the bag, and you want to apply 2 pounds of nitrogen per 1000 square feet, then this bag of fertilizer will cover 6,000 square feet
(12 pounds nitrogen in the bag) / (2 pounds nitrogen per 1000 sq. ft.) x (1000 square feet) = coverage of 6,000 sq. ft.
This same bag of fertilizer could be used to cover 12,000 square feet, but then you would be putting only one pound of nitrogen per 1000 square feet. This means you would need to fertilize often (once a month) to have a nice lawn.
Summary
Step 1 |
percent of nitrogen in the bag |
x |
weight of the bag |
= |
actual nitrogen present in the bag of fertilizer |
Step 2 |
amount of nitrogen in the nag of fertilizer / amount of nitrogen you want to put on 1,000 square feet |
x |
1,000 sq. ft. |
= |
area the bag of fertilizer will cover |
92. I applied an organic fertilizer to my lawn during the cool, fall weather but did not see any green-up until warm weather the following spring. Why?
In order for the grass to green-up, it had to be able to get the nitrogen from the soil. You applied the fertilizer too late, and the cool soil temperatures had reduced the activity of the soil micro organisms. Without these microorganisms (bacteria) to breakdown the fertilizer, the nitrogen wasn’t available to the plant. In the spring, when the soil warmed up, the bacteria broke down the fertilizer, and the nitrogen was then available. When you apply organic fertilizers, the soil temperature has to be at least 50 degrees F. or warmer before the bacteria are active enough to release the nitrogen.
93. I have heard that rain will pith up nitrogen and sulfur from the atmosphere, and this can act as a source of nutrients for plants. Is this true?
Rainfall does accumulate small amounts of nitrogen from the atmosphere and will deposit about pounds of nitrogen per year to 1 acre. The gaseous nitrogen in the atmosphere is converted to a water-soluble form whenever there is lightning, and the rain dissolves this nitrogen on its way to the earth. Sulfur and some of the other plant foods are brought back to the earth in the same manner as the nitrogen. However, they were usually released to the atmosphere in the form of smoke. In industrial areas, upward to 10 pounds of sulfur per acre can be carried to the earth by rainfall.
Prev: Grasses for Home Lawns
Next: Seeding Rates and Procedures